Hardness of Water
TOC/Help. Click to here expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
In this experiment, you will determine the “hardness” of a water sample by complexometric titration.
Learning Objectives
- Apply concepts of complexation chemistry to determine the calcium concentration of a hard water sample.
To cite this lab manual: “Hardness of Water”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Spring 2025.
Visual Abstract
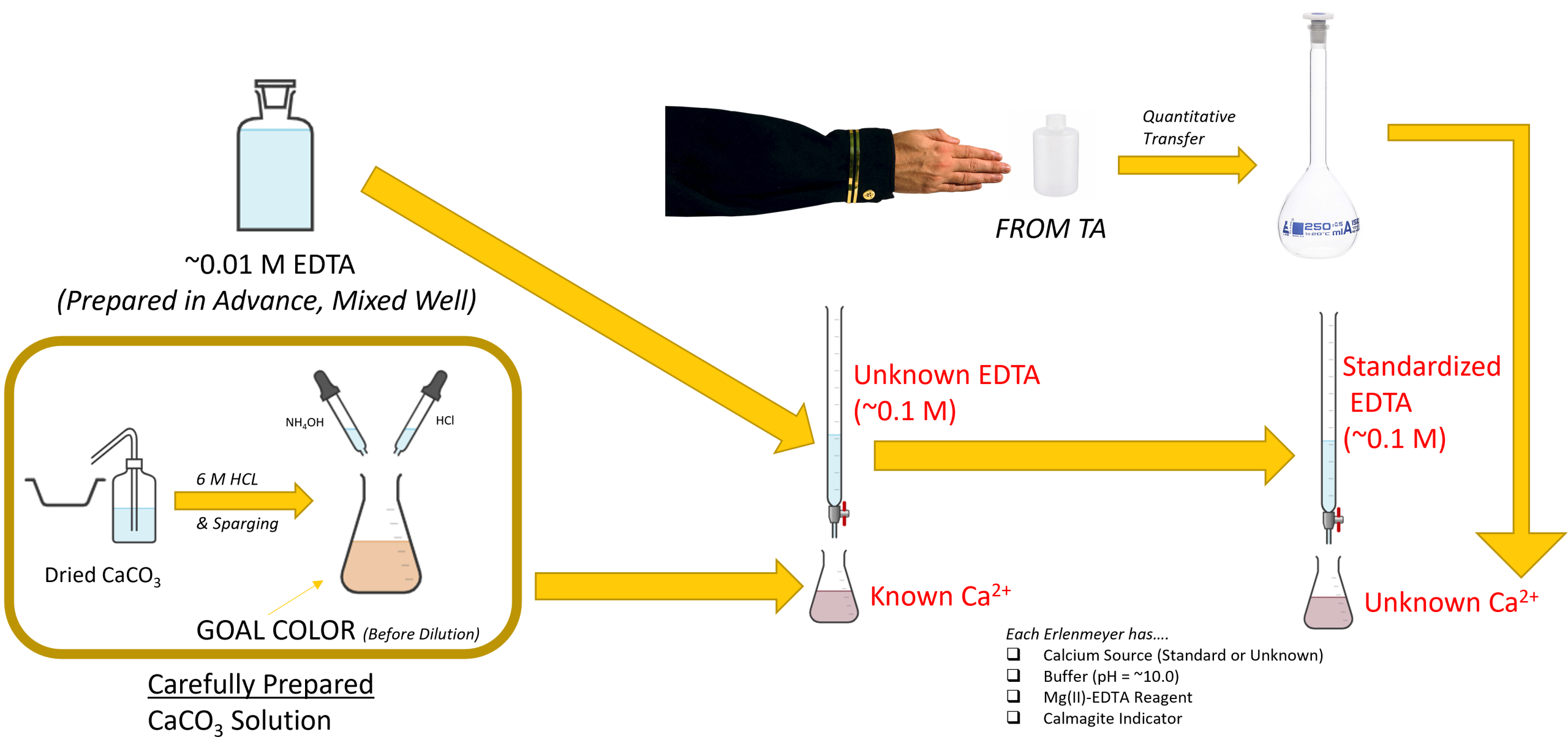

Background
Hard Water refers to water containing a considerable number of cations, which form precipitates with anions such as sulfate, carbonate, and soap. Because of its polarity and unique hydrogen-bonding properties, water is an excellent solvent. As water sits in contact with mineral solids found in other materials, such as pipes, reservoirs or simply the earth, metals such as aluminum, manganese, iron, and zinc are dissolved. The major cations involved in water hardness are Ca2+ and Mg2+. These ions are universally found dissolved in water.
The Madison area struggles with the problem of water hardness. Mineral deposits are formed by ionic reactions with cations in solution resulting in the formation of insoluble precipitates. For example, when hard water is heated, Ca2+ ions react with bicarbonate (HCO3¯) ions to form insoluble calcium carbonate (CaCO3). This precipitate, known as scale, coats the vessels in which water is heated, producing the mineral deposits on your dishes and in water pipes. In small quantities, these deposits are not harmful, but they may be frustrating to try to clean. More serious situations can occur in systems that boil water to produce steam. Hard water can cause deposits to build up inside pipes, eventually reducing or even blocking the flow of gas, building pressures inside the system to a dangerous level. Equally important—hard water can be blamed for bad hair days, as the soap complexes with these cations before it can get to the dirty deposits in your hair!
Hardness is numerically expressed in ppm CaCO3, even though other metal ions contribute to the measured hardness. This is due to tradition and to the fact that Ca is 95% the contributor. Generally, it is agreed that water containing less than 40 ppm CaCO3 is soft and that containing more than about 150 ppm CaCO3 is hard. Characterizing the gray area in-between depends on if you are in the business of selling water conditioners or fashionable mineral water.
The EDTA Titration
EDTA (Ethylenediaminetetraacetic acid) and its sodium salts is a complexing agent used to quantitatively determine metal ions in the first two columns of the periodic table. The procedure is faster and more convenient than by gravimetric analysis, and results are typically more precise than spectroscopic or electrochemical techniques. However, instrumental methods tend to be more sensitive in detecting concentrations of specific metals present, as well as detecting low concentrations of cations. Thus, the titration procedure described in this lab, is limited to non-specific analysis of metals at fairly high concentrations.
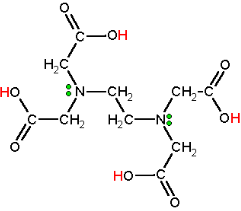
EDTA can exist in both acidic and basic forms, depending on the pH of the system. Figure 1 shows the structure of EDTA and the six active sites of the compound. We use the basic form Y4- for the complexation reaction. For this reason, the experiment is carried out at a basic pH. Choosing the appropriate pH can be tricky business, however, since at basic pH, some anions are forced out of solution by precipitation. You will explore this further in the prelaboratory exercises.
You will use calmagite indicator solution to detect the endpoint of the titration. Calmagite is an a,a’-dihydroxyazo dye and exists as a wine-red color in complexed form with calcium and magnesium ions at a pH of around 10.
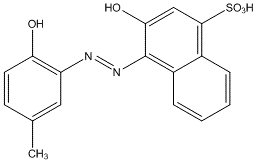
Notice in the procedure that a small amount of Mg-EDTA solution is added to the titration sample. This is done to sharpen the endpoint of the titration. Calmagite is a wine-red color when it is bound to metal cations. At the beginning of the titration, the sample (with only a small amount of EDTA added with the Mg cations) contains only free Ca2+ and metal complexed calmagite. As the titration progresses both Ca2+ and Mg2+ become captured by the EDTA added to solution. Once all free metal cations are bound, EDTA begins to compete with the metals bound to the calmagite indicator. The formation constant for Ca-Indicator is very weak (Kf =4.4 × 103) and as a result the color change observed with only Ca2+ in solution is very small. However, the Mg-indicator formation constant is much stronger, with Kf reported to be around 5 × 105. The final color change to a beautiful sky blue, with no hint of red or purple, is a result of the following reaction:
| MgIn¯(red) + Y4- ⇄ MgY2- + In3-(colorless) | (1) |
This reaction provides a sharp change of color. At the endpoint, calmagite is hydrolyzed:
| In3-(colorless) + H2O ⇄ HIn2-(blue) + OH¯ | (2) |
The hydrolysis reaction occurs rapidly, so visually observing the intermediate clear color is not possible. Thus, the endpoint is detected colorometrically by the change from wine-red to clear blue. Adding a small amount of Na2MgY ensures a sharp endpoint for this titration.

Lab Concept Video
click here to hide the video (for printing purpose).
Write down your observations or notes from the video in your lab notebook.
Pre-lab Work

Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
Key Takeaways
- Accurate and precise measurements are necessary to quantify the ‘hardness’ of water in this lab!
Extra Resources:

Prelaboratory Exercises
- You must prepare 0.01 M EDTA titrant at least one laboratory period beforehand. EDTA is slow to dissolve but eventually does so after a few hours. Calculate the amount of dihydrated disodium salt of EDTA (MW = 372.24 g/mol) needed to prepare 1 L of titrant.
- You will have to standardize the EDTA titrant by preparing a standard calcium solution. Analyzed reagent grade powdered CaCO3 is a good primary standard. It is also extremely hydrophilic and must be dried for 1-1.5 hours at 110 °C. Dried CaCO3 can be stored in your desiccator. You are asked in the procedure to weigh out 1.000 g of CaCO3 and dilute to a total volume of 1.000 L. What is the concentration of this solution? Report the answer in molarity.
- Write out the titration reactions for this experiment. What is the ratio of analyte/titrant?
- While performing the lab, your lab mate notices a systematic decrease in the amount of EDTA to reach the endpoint of the titration with CaCO3. Find a data table of those results below. Propose one reason why this may be happening and how to avoid it. Consider this: EDTA is a heavy molecule and acts slightly hydrophobic in solution. Its slow solubility is why you’re asked to make to EDTA standardizing solution the day before. A good explanation will consider the qualities mentioned, as well as the structure of EDTA in explaining why the solution of EDTA seems to change over the course of the experiment.
| Aliquot 1 | Aliquot 2 | Aliquot 3 | |
| Volume of Aliquot (mL) | 25.00 | 25.00 | 25.00 |
| Volume of EDTA to titrate CaCO3 Solution (mL) | 28.51 | 22.81 | 19.96 |

Before You Take The Quiz on Canvas
- Understand chelation and how EDTA reacts with metal cations.
- Understand the displacement titration technique, including how and why it is used in the experiment.
- Understand how EDTA is standardized using calcium carbonate.
- Be able to calculate the precise concentration of EDTA from a set of titration data—mass of calcium carbonate and the volume required to reach the end point.
- Be able to calculate the hardness of water from a set of raw data—precise concentration of EDTA and the volume required to reach the end point.

Experimental
-
- Prepare 1 L of 0.01 M standard EDTA titrant. Dissolve an accurately measured amount of the EDTA salt in DI water and mix well in a 1 L plastic (polyethylene) bottle. Because the titrant extracts hardness-producing cations from soft-glass containers, EDTA solution should not be stored in glass. This step should be done well before you’re ready to perform the experiment, since EDTA is slow to dissolve. You will standardize this solution against CaCO3, prepared in step 2.
- Prepare a standard calcium solution. Use reagent grade, dried CaCO3. Weigh 1.000 g of the anhydrous calcium carbonate into a beaker or weighing dish. Do not attempt to transfer the dry reagent directly into the flask. Instead, slurry it first with about 5 mL of deionized water and transfer it with the help of the wash bottle and a funnel set into the neck of a 500 mL Erlenmeyer flask. Do not use excessive amounts of water for the transfer; otherwise, the CaCO3 will not dissolve rapidly. Add 6 M HCl, a little at a time, until the CaCO3 has dissolved. Do not use more than 10 mL. Add 200 mL deionized water and air sparge for a few minutes to expel CO2. Add a few drops of methyl red indicator and adjust to the intermediate orange color by adding NH4OH or HCl, as required. Transfer quantitatively to a 1000 mL volumetric flask and fill to the mark with deionized water. This standard solution is equivalent to 1.00 mg /1.00 mL.
PRO TIP: Check the concentrations of the base (NH4OH) and acid (HCl) available. The point of this step is to get the standard CaCO3 solution to a neutral pH. This way the buffer will be more impactful at holding the pH at 10 while you titrate. Think about the amount of moles you would need to add to neutralize the acid you added. For example, if you added 10 mL of 6 M HCl to dissolve the CaCO3, you will need to add 60 mL of 1 M NH4OH. Add HCl using the principle “as much as necessary but as little as possible” will help with neutralizing the acid in the second step.
- Standardize the EDTA solution. Before filling the buret with EDTA, be sure to mix your EDTA solution well by inverting the bottle several times. It is a large molecule, and even though it is in aqueous form, the molecule tends to settle towards to bottom of the container when undisturbed.
- Pipet 25 mL of standard calcium solution into a 250 mL Erlenmeyer flask.
- Add 1 to 2 mL buffer solution. (This is used to set the pH of the mixture to 10. 0 to 10.1.)
- Add 10 drops of Mg (II) – EDTA reagent.
- Add 1 to 2 drops of the calmagite indicator.
- Perform your titrations by adding the standard EDTA titrant slowly, with continuous stirring, until the last reddish tinge disappears from the solution, adding the last few drops at 3 to 5 second intervals. At the end point, the solution is a crisp blue with no hue of wine red or purple. Daylight or a daylight fluorescent lamp is highly recommended; ordinary incandescent lights tend to produce a reddish tinge in the blue at the end point. Do not extend duration of titration beyond 5 min, measured from the time of the buffer addition. The absence of a sharp color change at the endpoint of the titration usually means that an inhibitor must be added at this point in the procedure, or that the indicator has deteriorated. Make sure you do three titrations; specify the indicator.
- Determine the hardness of an unknown sample. Give your laboratory instructor a clean 10 oz. plastic bottle properly labelled with your name and section number. When you receive your water sample, quantitatively transfer to a 250 mL volumetric flask and dilute to the mark with deionized water. Mix thoroughly. Pipet a 50 mL aliquot of unknown sample into a 250 mL Erlenmeyer flask and titrate as in Step 3. Adjust the procedure accordingly. Titrate two more 50 mL aliquots and calculate the “hardness” in ppm (mg/L) of CaCO3. Make sure you do three titrations.
Post-Lab Work Up

Results/Calculations
Fill out the answer sheet for this experiment completely. Use the answer sheet as a cover sheet to your notebook pages. This lab has an “accuracy” component of 10 points, thus pay attention to mixing all your solutions thoroughly, and check each other’s buret readings in lab to ensure you’re reading the burets correctly.

Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions. Any honest effort at answering the questions will be rewarded.
- A few drops of a Mg-EDTA reagent are used in this experiment to sharpen the endpoint. Suppose you were working with a lab partner, who misread the instructions to the experiment and accidentally pipetted 10 mL of this reagent to the solution. Must you start over with a new sample? If you must start over, explain what sort of error (systematic or random) you believe will be introduced to the measurement based on the mistake and what shift, if any, might result in the measured hardness of water of the sample.

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF file. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab, which should include answers to the post-lab questions and challenge questions.
The grading rubric can be found on Canvas.
References:
- Yappert, M. C., DuPre,’ D. B., J. Chem. Ed., 1997 74 p. 1422-142
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
