Introduction to Chromatography
This section serves to introduce two experiments, Gas Chromatography and HPLC (High Performance Liquid Chromatography). Some concepts discussed here may be useful in preparing for these laboratory experiments.
Chromatography Processor Operating Instructions
Chromatography is a widely used general method for separating the components of mixtures. It depends on differences in the partition coefficients of the components between a fixed (stationary) phase and mobile phase in contact with the fixed phase. The types of chromatography may be classified according to the nature of the mobile phase (liquid chromatography (LC) and gas chromatography (GC)), according to the type of equilibrium (ion-exchange chromatography) or some specific feature of the method (thin layer chromatography). In all chromatographic separations, the partition of components between the phases results in the separation of the components, as long as their partition coefficients (equilibrium constant for partition) are sufficiently different. The nature of the partition varies between types of chromatography, but chromatographic theory is sufficiently general so that all types can be described by it.
Consider a simplified picture of the chromatographic process. Let the mobile phase move by the stationary phase in a series of jumps, each corresponding to the introduction of an increment of mobile phase in the next region of phase contact. Let a solute be added to the system at the first contact region. Partition immediately occurs (Figure 1) so that K is satisfied.
[latex]K_A = \dfrac{[A_{stationary}]}{[A_{mobile}]} = \dfrac{C_s}{C_m}[/latex]
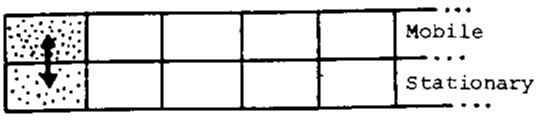
In the next instant, more pure mobile phase enters, displacing the (partially depleted) increment of mobile phase:
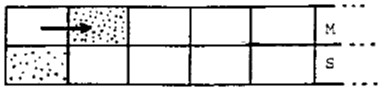
Then reequilibration occurs in each stage, resulting in Figure 3.
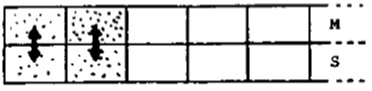
The next instant another increment of pure mobile phase enters the column, shifting each increment in the column down by one stage, and Reequilibrating. After the introduction of many such increments of mobile phase the column appears as shown in Figure 4, and a histogram can be made.
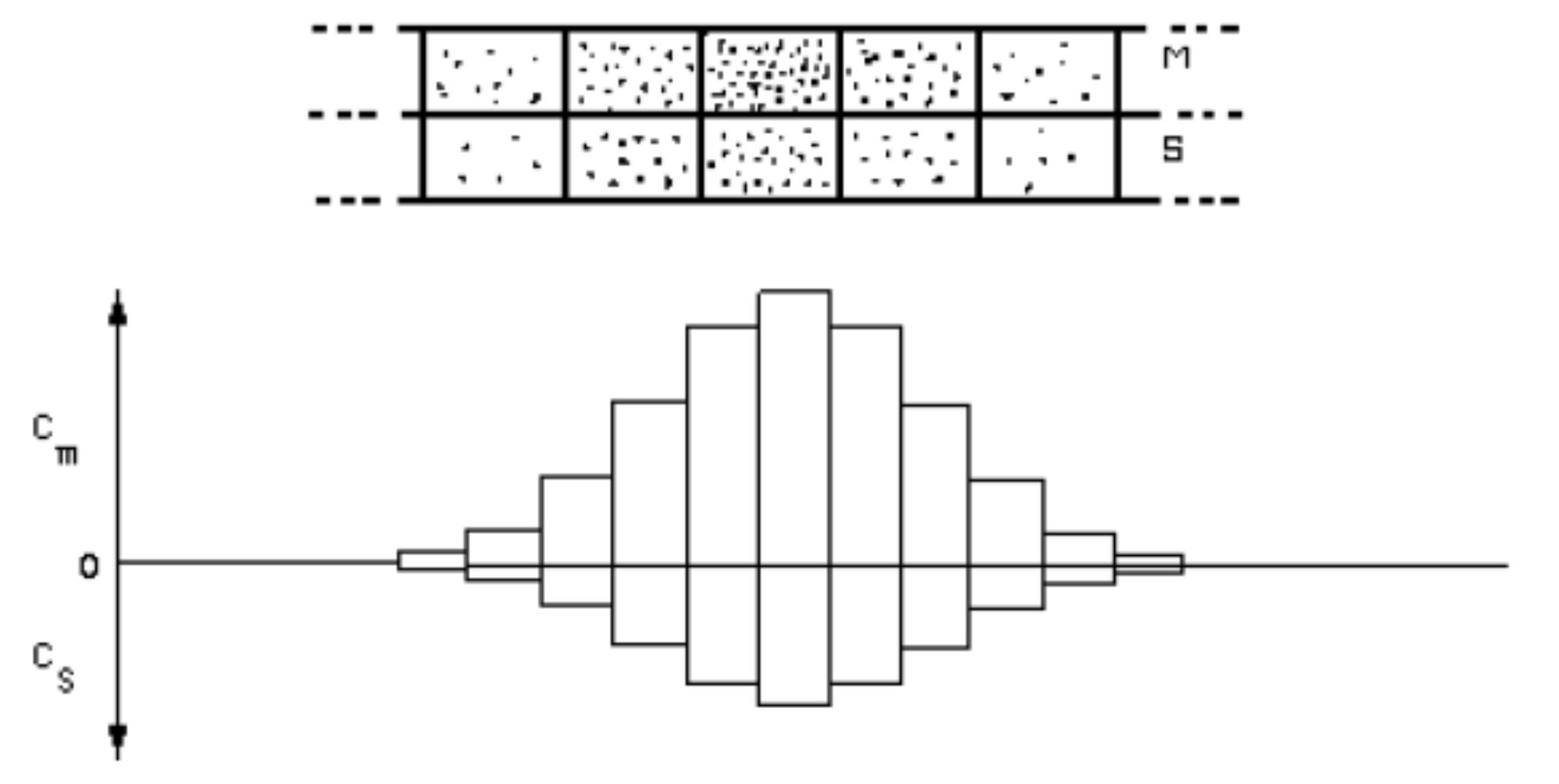
Now components with different K values will be concentrated at different stages. Such a case would appear as:
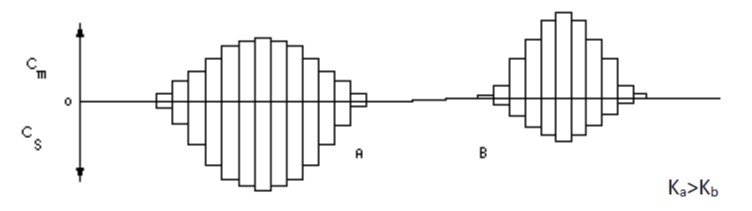
Note that a large value of K implies increased concentration in the stationary phase, or, alternatively, decreased concentration in the mobile phase. Therefore less of the component moves through the column in any increment and in fact the entire component zone moves more slowly when K is large. A difference in K between two components results in a difference in the rates of movement of the components through the column.
Now consider the case when the stage length in a column is small compared to the overall column length. We can plot the mobile phase concentration of solute as a function of column length (L):
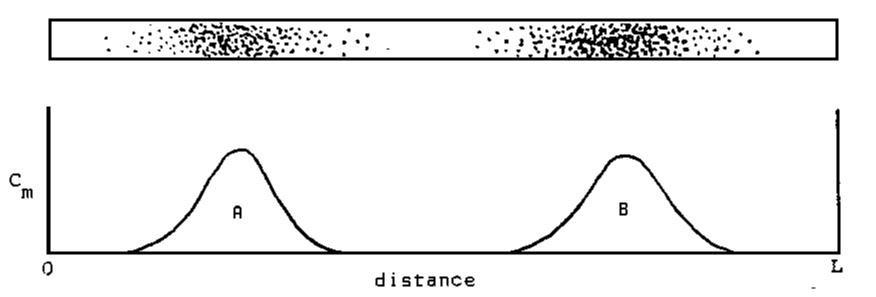
When a given length of time has elapsed after sample introduction, the addition of mobile phase may be interrupted and the component zones found. If the zones are colored materials and if the column sides are transparent, visual detection is possible. In thin-layer chromatography, the stationary phase is a sheet of material (cellulose, silica, or alumina on aluminum, glass or plastic) and detection is usually visual. Often the solute zones need to be rendered visible by application of a "visualization reagent" that reacts with the components resulting in a colored product.
In certain cases (HPLC and GC), examination of the column itself is very difficult and a slightly modified method is used, that of elution. If components have different partition coefficients, then they must migrate at differing rates through the column. Since the column is of finite length, mobile phase containing the solutes must emerge from the end of the column and we have only to measure the solute concentration in the column effluent as a function of the volume of effluent in order to locate the solute zones.
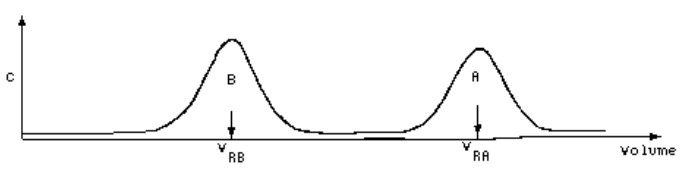
Note that B has a smaller K value than does A, hence is relatively more concentrated in the mobile phase in any stage, and therefore traverses the column in a smaller volume of mobile phase. The volume required to elute B is clearly dependent on KB, the partition coefficient for solute B.
If the volume flow rate is held constant, then Fig. 7 may be plotted as:
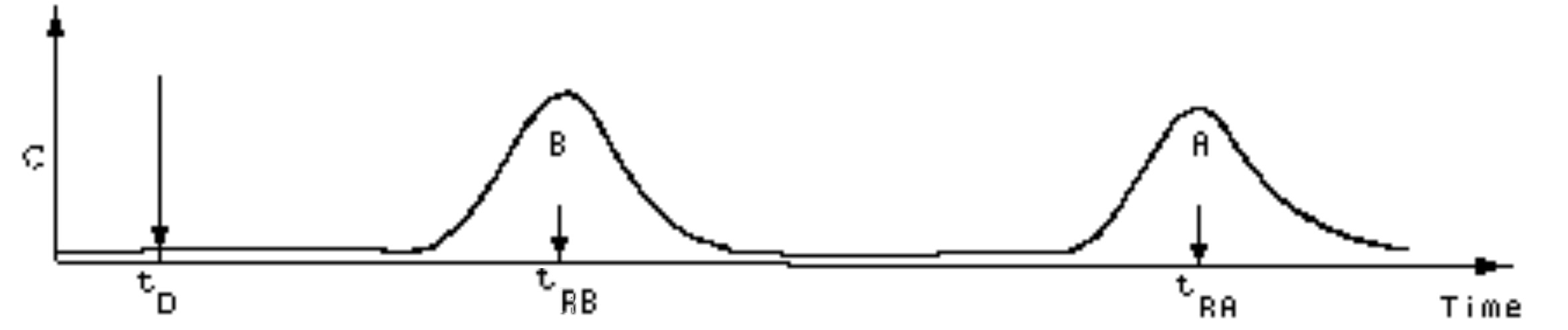
where tRB is the retention time of B corresponding to components with partition coefficient KB, and tD is the time of elution for a component whose K is zero. Then
F(tRB − tD) = VRB − VD ≡ V'RB
where F is the volume flow rate of mobile phase through the column, VD is the "column void space" (i.e., F·tD, or the volume required to elute a component whose K is 0) and V'RB is defined as the corrected retention volume (or "corrected volume of elution") of component B.
It can be shown that
V'RB = β·KB
where β is a constant for a given column but is independent of the nature of the eluted component, hence β is independent of K.
Several items are necessary to perform a chromatographic experiment:
- a supply of mobile phase
- a column or support containing the stationary phase, and through which the mobile phase can flow
- a sample introduction system, to place the sample in the flowing mobile phase ahead of the column
- a detection system, to monitor component concentrations in the effluent
- a recording system to make a permanent record of the concentration of solute vs. time or volume of effluent
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
