Preparation of Standard HCl Solution (Wet) — test ground
Preparation of Standard HCl Solution (Wet)
Need Help?
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).

Abstract
Lab Description: Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. We use these basic titrations to assess your accuracy and precision in performing the analytical measurements, and introduce the use of statistics as applied to analytical chemistry.
To cite this lab manual: “Preparation of a Standard HCl Solution – 327 Edition”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW-Madison, Summer 2023.
Visual Abstract

Background
Standard solutions of hydrochloric acid are usually prepared by diluting the commercially available concentrated acid and then standardizing against primary standard base. This method is used because of the difficulty in obtaining an accurate value for the concentration of the concentrated acid.
Tris-(hydroxymethyl)aminomethane also known as “THAM” is an excellent primary standard base. THAM has a molecular weight of 121.14 g/mole corresponding to the formula:
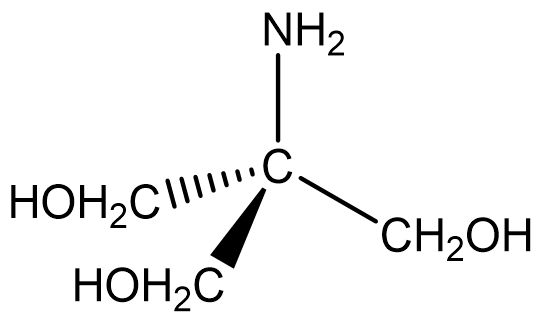
You can see from the structure that THAM can be regarded as a derivative of ammonia, given the basic nitrogen atom. It is a good primary standard because it is non-hygroscopic, does not absorb carbon dioxide from air, is stable both as the solid and in aqueous solution, can be prepared in very pure form, can be dried at 100-105 °C without decomposition, and reacts quickly and stoichiometrically with hydronium ion (H+).
Sodium carbonate is another excellent primary standard. It is available in very pure form except for traces of sodium bicarbonate. If dried at 160 °C, this impurity is removed. The disadvantage of using sodium carbonate is that as the end point is reached the solution contains a large amount of carbon dioxide and a small amount of sodium bicarbonate which acts as a buffer and the end point is not sharp. The sharpness of the end point can be greatly improved by stopping the titration at the point where the indicator just begins to change color and removing the carbon dioxide by either air-sparging or boiling and then completing the titration. This additional manipulation of the solution introduces the possibility of additional sources of error.
An analytical chemist must think carefully about factors that may affect the quality of a solution. For example, aged or contaminated reagents may prove useless or even dangerous while performing an analysis. Dirty glassware can add contaminants that interfere with the expected reaction of investigation. And since an analytical chemist often times is interested in quantifying amounts of substances present in a sample, it is extremely important to know how to prepare and standardize solutions used in a particular analysis. Your TA has described important factors to consider regarding laboratory technique while preparing solutions. You should also be familiar with the reaction taking place between the chemicals present in solution. You should know, for example, what functional groups or atoms are actively involved in the reaction.
But how do you evaluate your data once you finish your experiment? How do you report your data in a way that is meaningful? The answer is to use statistics to report your experimental measurements. Statistics allows the analytical chemist to report how accurate and/or precise a measurement is and also allows a means to reject a measurement that seems out of line or incorrect. A standard practice for reporting the answer to any quantitative measurement is collect the average of at least three measurements, and report the variation or precision of the result by including a standard deviation.
In this investigation, you will standardize a solution that is approximately 0.1 M HCl with the primary standard THAM. You will create this solution of HCl by quantitative transfer of a unique volume dispensed to you by your TA into a 1 L flask. The assessment for this lab involves the accuracy and precision of the result, which is a reflection of the careful work taken to make the solutions and the measurements.
Lab Concept Video
Pre-lab Work
 Lab Skills
Lab Skills
Review these lab skills videos prior to lab.
Key Takeaways
- Titrations take a lot of patience and practice to hit the end point correctly.
- By using volumetric glassware, we can improve the accuracy and precision of our measurements.

Prelaboratory Exercises
Read the procedure and outline the procedure in your notebook. This should be done in such a way that you need not bring loose printed pages or your manual to class. You should complete the exercises below prior to the lab period. Incorporate the calculations into the body of your written procedure.
- Write out the chemical reaction for the titration of THAM with HCl. Draw the structure for THAM and indicate which atom participates in the reaction.
- You will use a 50 ml buret to titrate with. HCl will be placed in the buret. You would like to use the optimum range of the buret for the titration, which is between 25–40 ml. Using more than 40 ml, you run the risk of having to refill the buret for the titration. This adds a significant amount of error to your measurement. Using less than 10 ml may mean the reagents are too concentrated, leading to passing the endpoint of the titration hastily. Also, by using less than 10 ml, the tolerance label for the buret no longer applies, since the number is meant for the entire volume. You could improve the resolution of your measurement in this case by using a 10 ml buret, or by diluting your initial solutions. Choose an optimum volume of HCl that you would like to use for the titration. Then, calculate the grams of THAM you must weigh out that will completely react with the HCl present in that volume. If you dissolve THAM into 50 mL of DI water, what is the molarity of the solution?
- You’re instructed later on to make 250 mL of the THAM solution for standardizing the HCl. Calculate the grams of THAM needed to make 250 mL of the required solution.
 Before You Take The Quiz on Canvas
Before You Take The Quiz on Canvas
- Know the purpose of a primary standard and understand the process of standardization (when and why it is required).
- Understand the chemistry and stoichiometry of the titration reactions.
- Be able to calculate how much THAM or KHP should be used for the standardization given a set of initial parameters—approximate concentration of HCl/NaOH and a target end point volume.
- Be able to calculate the precise concentration of HCl/NaOH given a set of titration data—mass of KHP/THAM used, and the volume required to reach the end point.

Experimental
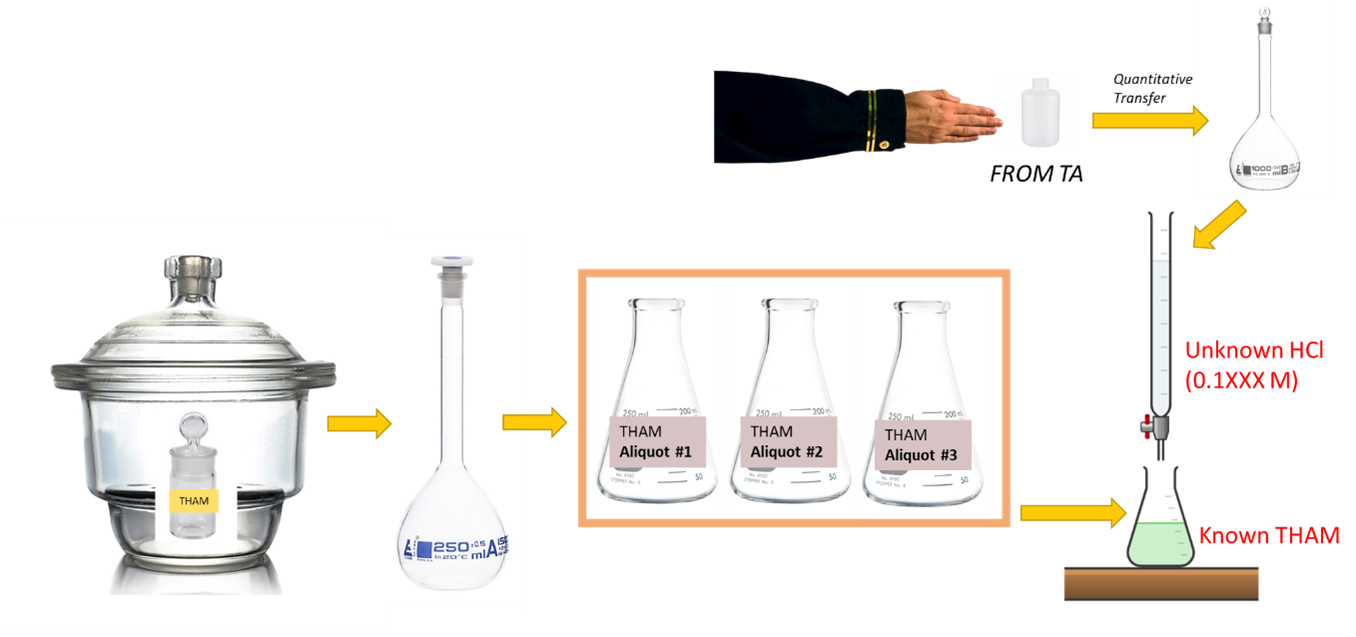
- Prepare unknown hydrochloric acid solution. Give your instructor a clean 10 oz. plastic bottle properly labeled with your name and section number. When you receive your unknown solution, quantitatively transfer it to your 1000 mL volumetric flask. Dilute to the mark with deionized water and mix thoroughly.
- Weigh out the grams of THAM you calculated in the prelab exercise (3) into a clean weighing bottle and dry at 100-105 °C for 1-2 hours. A hotter oven will cause the THAM to melt. Cool in your desiccator. Weighing by difference, transfer dried THAM to a small beaker.
If you have already dried some THAM (and stored it in your desiccator), you may weigh out the amount of THAM you need from that pre-dried sample!
Make sure to record the final mass. Then transfer quantitatively to your calibrated 250 mL volumetric flask. Swirl the flask until the THAM is dissolved, dilute to the mark with deionized water and mix well. - Standardize the solution using the procedure described below. Report the average concentration from three titrations to your laboratory instructor, in order to check how close you are to the expected answer. The concentration of this solution should be in the vicinity of 0.1 M.
- Pipet a 50 mL aliquot of THAM solution into a 250 mL Erlenmeyer flask. Add 2 drops of bromocresol green indicator. Titrate with your unknown HCl solution.
BROMOCRESOL GREEN CHANGES FROM BLUE, THROUGH GREEN, TO YELLOW. THE PROPER STOPPING POINT IS AT THE INTERMEDIATE GREEN COLOR–THAT CORRESPONDS TO A pH OF 4.7. - Titrate at least two more 50 mL aliquots (for a total of 3 at a minimum). Calculate the average molarity of the HCl solution.
Titration Procedure
- Prepare and fill the buret using the procedure previously described in the Good Lab Practices experiment and video. Fix the buret to a buret clamp, and attached the apparatus to a ring stand. Check you have removed the bubble from the buret! Record the initial volume.
- Have on hand a wash bottle, Erlenmeyer flasks, the standard THAM solution, receiving beakers for your solutions, a waste beaker, Kimwipes, and droppers.
- Pipet a 50.0 mL aliquot of the THAM standard solution to one of the Erlenmyer flasks. Add two to three drops of Bromocresol Green indicator.
- Titrate the sample to the intermediate green endpoint. Record the final volume.
- Titration tips:
- You can perform a “range-finding” titration by quickly adding NaOH until you read the endpoint. This replicate doesn’t count for your 3 analytical trials but can give you an estimate to what volume you will use on the buret!
- If you are not sure whether you are at the endpoint, you can write down the volume, add a drop, and then re-evaluate. If the additional drop pushes the color too yellow, then you can use the data already recorded for your calculation.
- If you have a ½ drop hanging off the buret tip, you can rinse it off with a small wash of DI water. The moles in the solution are what matters in measuring the endpoint. The additional volume will not impact the number of moles in the beaker. In a similar way, you can add a ½ drop by intentionally letting a small drop develop on the buret tip, and then wash the drop into your flask using the DI water wash bottle.
- Once you have identified the endpoint, for your next trials, you can collect the additional data quickly by dispensing 80% of the volume with the stopcock fully open, and then slow to adding a drop at a time just before you reach the endpoint.
- Use the buret reading cards to create a clearer view of the meniscus in the buret. Placing a white sheet of paper under the flask sometimes helps in making the colored endpoints easier to see as well. Have You Mastered the Four Significant Figure Lab Technique?
Post-Lab Work Up
Results/Calculations
- Calculate the average value of the three molarities you have calculated for your HCl. Calculate the sample standard deviation (s) for these three results.
- Calculate the Relative Standard Deviation (RSD) =
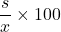 .
.
Have You Mastered the Four Significant Figure Lab Technique?
Your grade is dependent on both the accuracy and precision of your results. Your TA will discuss with you the importance of rounding correctly and using proper rules for significant figures. The results from this experiment should be significant to four figures. How can you tell this is true? In general, the last significant figure in your final answer (which is the average of at least three titrations) will be where the first digit appears in your standard deviation (or uncertainty) calculation.
If you have mastered this technique the RSD should be less than 0.3 %. If it is not, you should think about repeating the standardization as it seems your technique lacks precision.
 Challenge Questions
Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions! Any honest effort at answering the questions will be rewarded.
- For a primary standard to be effective, it must have a specific set of characteristics. If any of these are missing, there will be an error in your standardization. Explain the challenge imposed by each situation below, and propose how you would account for each of these cases.
- Your primary standard was only 75% pure (assuming the other 25% was non-reactive).
- Your primary standard was only stable in solution for 1 hour.
- Your primary standard absorbed water rapidly before you could use it (even after being in the oven).
- Your primary standard has a low molecular weight.
- From the pre-lab videos and your experience in the lab, rationalize what would happen if you used phenolphthalein instead of bromocresol green for standardizing HCl.
- Suggest an alternative method we can use to determine the equivalence point if you could not use a colorimetric indicator.

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab
The grading rubric can be found on Canvas.

