Stoichiometry
Conservation of Matter
The law of conservation of matter summarizes many scientific observations about matter: It states that there is no detectable change in the total quantity of matter present when matter converts from one type to another (a chemical change) or changes among solid, liquid, or gaseous states (a physical change). What does this mean for chemistry? In any chemical change, one or more initial substances change into a different substance or substances. Both the initial and final substances are composed of atoms because all matter is composed of atoms. According to the law of conservation of matter, matter is neither created nor destroyed, so we must have the same number and type of atoms after the chemical change as were present before the chemical change.
Although this conservation law holds true for all conversions of matter, convincing examples are few and far between because, outside of the controlled conditions in a laboratory, we seldom collect all of the material that is produced during a particular conversion. For example, when you eat, digest, and assimilate food, all of the matter in the original food is preserved. But because some of the matter is incorporated into your body, and much is excreted as various types of waste, it is challenging to verify by measurement.
When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. Consider as an example the reaction between one methane molecule (CH4) and two diatomic oxygen molecules (O2) to produce one carbon dioxide molecule (CO2) and two water molecules (H2O). The chemical equation representing this process is provided in the upper half of Figure 1, with space-filling molecular models shown in the lower half of the figure.
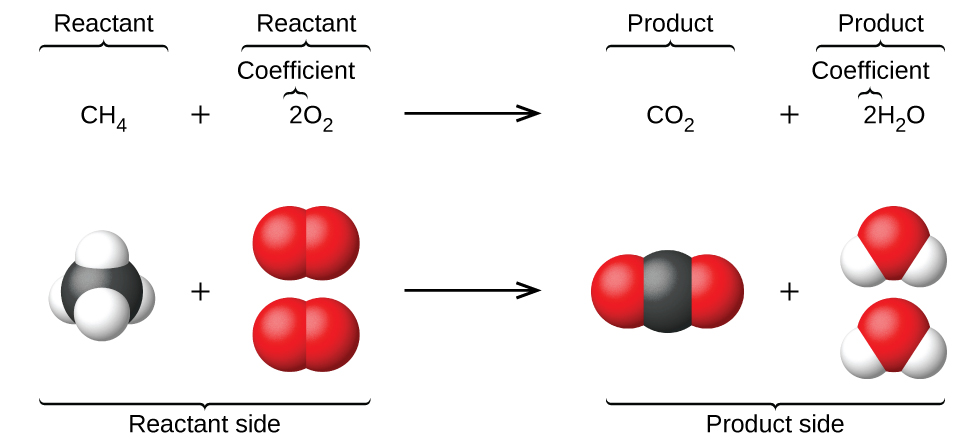
This example illustrates the fundamental aspects of any chemical equation:
- The substances undergoing reaction are called reactants, and their formulas are placed on the left side of the equation.
- The substances generated by the reaction are called products, and their formulas are placed on the right side of the equation.
- Plus signs (+) separate individual reactant and product formulas, and an arrow (⟶) separates the reactant and product (left and right) sides of the equation.
- The relative numbers of reactant and product species are represented by coefficients (numbers placed immediately to the left of each formula). A coefficient of 1 is typically omitted.
It is common practice to use the smallest possible whole-number coefficients in a chemical equation, as is done in this example. Realize, however, that these coefficients represent the relative numbers of reactants and products, and, therefore, they may be correctly interpreted as ratios.
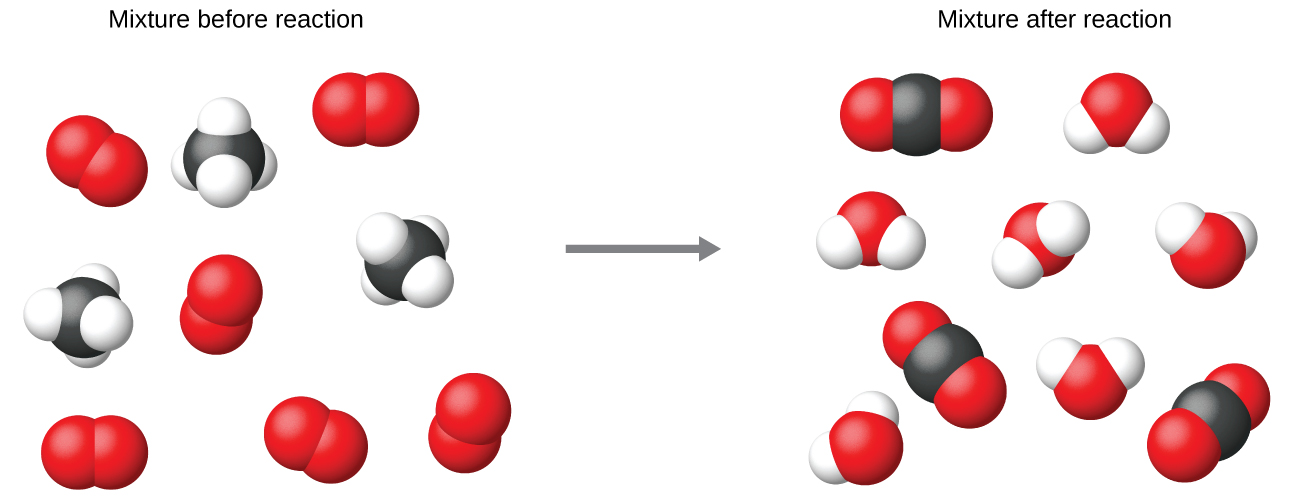
Methane and oxygen react to yield carbon dioxide and water in a 1:2:1:2 ratio. This ratio is satisfied if the numbers of these molecules are, respectively, 1-2-1-2, or 2-4-2-4, or 3-6-3-6, and so on (Figure 2). Likewise, these coefficients may be interpreted with regard to any amount (number) unit, and so this equation may be correctly read in many ways, including:
- 1 methane molecule and 2 oxygen molecules react to yield 1 carbon dioxide molecule and 2 water molecules.
- 1 dozen methane molecules and 2 dozen oxygen molecules react to yield 1 dozen carbon dioxide molecules and 2 dozen water molecules.
- 1 mole of methane molecules and 2 moles of oxygen molecules react to yield 1 mole of carbon dioxide molecules and 2 moles of water molecules.
Balancing Equations
The chemical equation described above is a balanced equation, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. It may be confirmed by simply summing the numbers of atoms on either side of the arrow and comparing these sums to ensure they are equal. Note that the number of atoms for a given element is calculated by multiplying the coefficient of any formula containing that element by the element’s subscript in the formula. If an element appears in more than one formula on a given side of the equation, the number of atoms represented in each must be computed and then added together. For example, both product species in the example reaction, CO2 and H2O, contain the element oxygen, and so the number of oxygen atoms on the product side of the equation is 2 (from CO2) + 2 (from 2 H2O) = 4 product oxygen atoms. Thus, we’ll need two O2 molecules as reactants to similarly have 4 reactant oxygen atoms.
The equation for the reaction between methane and oxygen to yield carbon dioxide and water is confirmed to be balanced per this approach, as shown here:
CH4 + 2 O2 ⟶ CO2 + 2 H2O
| Element | Reactants | Products | Balanced? |
| C | 1 × 1 = 1 | 1 × 1 = 1 | 1 = 1, yes |
| H | 4 × 1 = 4 | 2 × 2 = 4 | 4 = 4, yes |
| O | 2 × 2 = 4 | (1 × 2) + (2 × 1) = 4 | 4 = 4, yes |
A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. This process is represented qualitatively by an unbalanced chemical equation:
H2O ⟶ O2 + H2
Comparing the number of H and O atoms on either side of this equation confirms its imbalance:
| Element | Reactants | Products | Balanced? |
| H | 1 × 2 = 2 | 1 × 2 = 2 | 2 = 2, yes |
| O | 1 × 1 = 1 | 1 × 2 = 2 | 1 ≠ 2, no |
The numbers of H atoms on the reactant and product sides of the equation are equal, but the numbers of O atoms are not. To achieve balance, the coefficients of the equation may be changed as needed. Keep in mind, of course, that the formula subscripts define, in part, the identity of the substance, and so these cannot be changed without altering the qualitative meaning of the equation. For example, changing the reactant formula from H2O to H2O2 would yield balance in the number of atoms, but doing so also changes the reactant’s identity (it’s now hydrogen peroxide and not water). The O atom balance may be achieved by changing the coefficient for H2O to 2.
2 H2O ⟶ O2 + H2
| Element | Reactants | Products | Balanced? |
| H | 2 × 2 = 4 | 1 × 2 = 2 | 4 ≠ 2, no |
| O | 2 × 1 = 2 | 1 × 2 = 2 | 2 = 2, yes |
The H atom balance was upset by this change, but it is easily reestablished by changing the coefficient for the H2 product to 2.
| Element | Reactants | Products | Balanced? |
| H | 2 × 2 = 4 | 2 × 2 = 4 | 4 = 4, yes |
| O | 2 × 1 = 2 | 1 × 2 = 2 | 2 = 2, yes |
These coefficients yield equal numbers of both H and O atoms on the reactant and product sides, and the balanced equation is, therefore:
2 H2O ⟶ O2 + 2 H2
Notice that in all these equations, the charge is also balanced on both the reactant and product sides. In the previous example, there is a total of zero charge on both sides of the equation since H2O, O2, and H2 are all neutral molecules. This will become important later when we start writing equations with ions—the total charge on the reactant side must be equal to the total charge on the product side since electrons cannot be lost in a chemical equation.
Example 1: Balancing Chemical Equations
Write a balanced equation for the reaction of molecular nitrogen (N2) and oxygen (O2) to form dinitrogen pentoxide.
Solution
First, write the unbalanced equation.
N2 + O2 ⟶ N2O5
Next, count the number of each type of atom present in the unbalanced equation.
| Element | Reactants | Products | Balanced? |
| N | 1 × 2 = 2 | 1 × 2 = 2 | 2 = 2, yes |
| O | 1 × 2 = 2 | 1 × 5 = 5 | 2 ≠ 5, no |
Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. To balance the number of oxygen atoms, a reasonable first attempt would be to change the coefficients for the O2 and N2O5 to integers that will yield 10 O atoms (the least common multiple for the O atom subscripts in these two formulas).
N2 + 5 O2 ⟶ 2 N2O5
| Element | Reactants | Products | Balanced? |
| N | 1 × 2 = 2 | 2 × 2 = 4 | 2 ≠ 4, no |
| O | 5 × 2 = 10 | 2 × 5 = 10 | 10 = 10, yes |
The N atom balance has been upset by this change; it is restored by changing the coefficient for the reactant N2 to 2.
2 N2 + 5 O2 ⟶ 2 N2O5
| Element | Reactants | Products | Balanced? |
| N | 2 × 2 = 4 | 2 × 2 = 4 | 4 = 4, yes |
| O | 5 × 2 = 10 | 2 × 5 = 10 | 10 = 10, yes |
The numbers of N and O atoms on either side of the equation are now equal, and so the equation is balanced.
Check Your Learning
Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. (Hint: Balance oxygen last, since it is present in more than one molecule on the right side of the equation.)
Answer
2 NH4NO3 ⟶ 2 N2 + O2 + 4 H2O
It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. When balance is achieved, all the equation’s coefficients may then be multiplied by a whole number to convert the fractional coefficients to integers without upsetting the atom balance. For example, consider the reaction of ethane (C2H6) with oxygen to yield H2O and CO2, represented by the unbalanced equation:
C2H6 + O2 ⟶ CO2 + H2O
Following the usual inspection approach, one might first balance C and H atoms by changing the coefficients for the two product species, as shown:
C2H6 + O2 ⟶ 2 CO2 + 3 H2O
This results in seven O atoms on the product side of the equation, an odd number—no integer coefficient can be used with the O2 reactant to yield an odd number, so a fractional coefficient, 7/2, is used instead to yield a provisional balanced equation:
C2H6 + ![]() O2 ⟶ 2 CO2 + 3 H2O
O2 ⟶ 2 CO2 + 3 H2O
A conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by 2:
2 C2H6 + 7 O2 ⟶ 4 CO2 + 6 H2O
Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients. Although the equation for the reaction between molecular nitrogen and molecular hydrogen to produce ammonia is, indeed, balanced,
3 N2 + 9 H2 ⟶ 6 NH3
the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. Dividing each coefficient by the greatest common factor, 3, gives the preferred equation:
N2 + 3 H2 ⟶ 2 NH3
Additional Information in Chemical Equations
The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids, l for liquids, g for gases, and aq for substances dissolved in water (aqueous solutions are solutions where water is the solvent). These notations are illustrated in the example equation here:
2 Na(s) + 2 H2O(ℓ) ⟶ 2 NaOH(aq) + H2(g)
This equation represents the reaction that takes place when sodium metal is placed in water. The solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid in pure form, but readily dissolved in water).
Stoichiometry
A balanced chemical equation provides a great deal of information in a very succinct format. Chemical formulas provide the identities of the reactants and products involved in the chemical change, allowing classification of the reaction. Coefficients provide the relative numbers of these chemical species, allowing a quantitative assessment of the relationships between the amounts of substances consumed and produced by the reaction. These quantitative relationships are known as the reaction’s stoichiometry, a term derived from the Greek words stoicheion (meaning “element”) and metron (meaning “measure”). Here, the use of balanced chemical equations for various stoichiometric applications is explored.
The general approach to using stoichiometric relationships is similar in concept to the way people go about many common activities. Food preparation, for example, offers an appropriate comparison. A recipe for making eight pancakes calls for 1 cup pancake mix, ¾ cup milk, and one egg. The “equation” representing the preparation of pancakes per this recipe is
1 cup mix + ¾ cup milk + 1 egg → 8 pancakes
If two dozen pancakes are needed for a big family breakfast, the ingredient amounts must be increased proportionally according to the amounts given in the recipe. For example, the number of eggs required to make 24 pancakes is
24 pancakes × ![]() = 3 eggs
= 3 eggs
Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant, or to yield a given amount of product, and so forth. The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. To illustrate this idea, consider the production of ammonia by reaction of hydrogen and nitrogen:
N2(g) + 3 H2(g) → 2 NH3(g)
This equation shows ammonia molecules are produced from hydrogen molecules in a 2:3 ratio, and stoichiometric factors may be derived using any amount (number) unit:
![]() or
or ![]() or
or ![]()
These stoichiometric factors can be used to compute the number of ammonia molecules produced from a given number of hydrogen molecules, or the number of hydrogen molecules required to produce a given number of ammonia molecules. Similar factors may be derived for any pair of substances in any chemical equation.
Example 2: Moles of Reactant Required in a Reaction
How many moles of I2 are required to react with 0.429 mol of Al according to the following equation (see Figure 1)?
2 Al(s) + 3 I2(s) ⟶ 2 AlI3(s)

Solution
Referring to the balanced chemical equation, the stoichiometric factor relating the two substances of interest is ![]() . The molar amount of iodine is derived by multiplying the provided molar amount of aluminum by this factor:
. The molar amount of iodine is derived by multiplying the provided molar amount of aluminum by this factor:

mol I2 = 0.429 mol Al × ![]() = 0.644 mol I2
= 0.644 mol I2
Check Your Learning
How many moles of Ca(OH)2 are required to react with 1.36 mol of H3PO4 to produce Ca3(PO4)2 according to the following equation?
3 Ca(OH)2(aq) + 2 H3PO4(aq) → Ca3(PO4)2(s) + 6 H2O(ℓ)
Answer
2.04 mol
Example 3: Number of Product Molecules Generated by a Reaction
How many carbon dioxide molecules are produced when 0.75 mol of propane is combusted according to this equation?
C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(ℓ)
Solution
The approach here is the same as for Example 1, though the absolute number of molecules is requested, not the number of moles of molecules. This will simply require use of the moles-to-numbers conversion factor, Avogadro’s number.
The balanced equation shows that carbon dioxide is produced from propane in a 3:1 ratio:
![]()
Using this stoichiometric factor, the provided molar amount of propane, and Avogadro’s number,

![]() = 1.4 × 1024 CO2 molecules
= 1.4 × 1024 CO2 molecules
Check Your Learning
How many NH3 molecules are produced by the reaction of 4.0 mol of Ca(OH)2 according to the following equation:
(NH4)2SO4(aq) + Ca(OH)2(aq) → 2 NH3(g) + CaSO4(s) + 2 H2O(ℓ)
Answer
4.8 × 1024 NH3 molecules
These examples illustrate the ease with which the amounts of substances involved in a chemical reaction of known stoichiometry may be related. Directly measuring numbers of atoms and molecules is, however, not an easy task, and the practical application of stoichiometry requires that we use the more readily measured property of mass.
Example 4: Relating Masses of Reactants and Products
What mass of sodium hydroxide, NaOH, would be required to produce 16 g of the antacid milk of magnesia [magnesium hydroxide, Mg(OH)2] by the following reaction?
MgCl2(aq) + 2 NaOH(aq) → Mg(OH)2(s) + NaCl(aq)
Solution
The approach used previously in Example 1 and Example 2 is likewise used here; that is, we must derive an appropriate stoichiometric factor from the balanced chemical equation and use it to relate the amounts of the two substances of interest. In this case, however, masses (not molar amounts) are provided and requested, so additional steps are required. The calculations required are outlined in this flowchart:
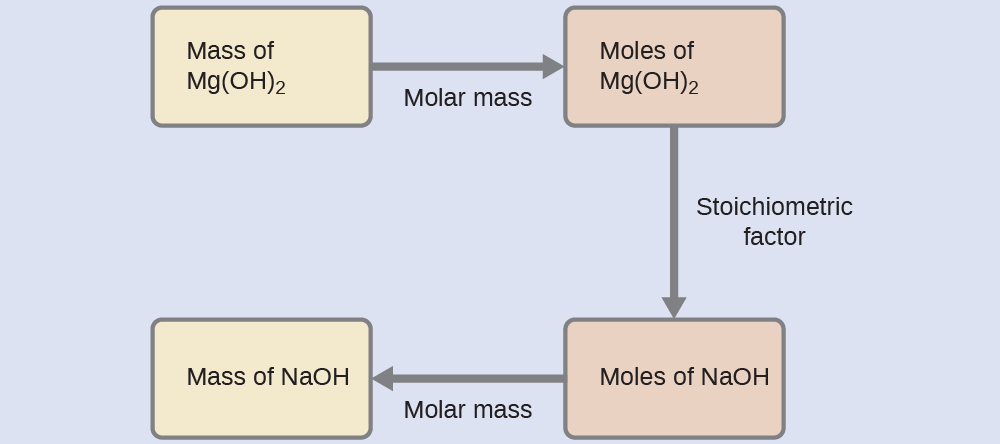
![]() = 22 g NaOH
= 22 g NaOH
Check Your Learning
What mass of gallium oxide, Ga2O3, can be prepared from 29.0 g of gallium metal? The equation for the reaction is:
4 Ga(s) + 3 O2(g) → 2 Ga2O3(s)
Answer
39.0 g
Example 5: Relating Masses of Reactants
What mass of oxygen gas, O2, from the air is consumed in the combustion of 702 g of octane, C8H18, one of the principal components of gasoline?
2 C8H18(ℓ) + 25 O2(g) → 16 CO2(g) + 18 H2O(ℓ)
Solution
The approach required here is the same as for the Example 3, differing only in that the provided and requested masses are both for reactant species.
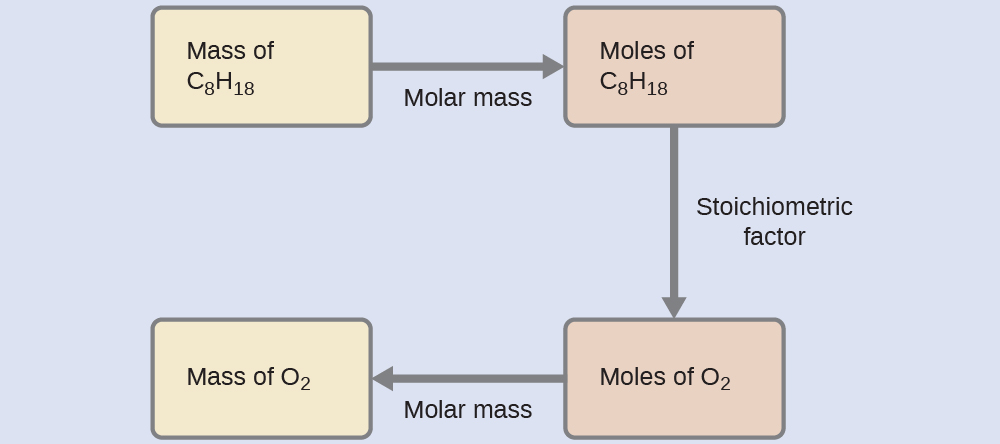
![]() = 2.46 × 103 g O2
= 2.46 × 103 g O2
Check Your Learning
What mass of CO is required to react with 25.13 g of Fe2O3 according to the equation:
Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g)
Answer
13.22 g

