Ionic Compounds
Ionic Bonding
Our discussion up to now has centered on types of bonds that involve valence electrons being shared between (or more correctly being fought over – attracted to the opposite nuclei) different atoms. We have seen that we can consider electron density to be equally distributed between the bonding atoms, or that it may be distorted by being attracted to the more electronegative atom. What we have not looked at yet is the extreme case of this kind of distortion, in which the valence electrons are attracted so much by the electronegative atom that they are transferred completely. This kind of bonding is called ionic bonding (as you are almost certainly already aware).
Let us take a look at some common ionic compounds and see if we can make some sense of their properties from a consideration of their atomic-molecular structure. For the sake of simplicity we will confine ourselves (for the moment) to binary compounds (compounds with only two elements in them.) The most familiar of these compounds is sodium chloride (NaCl), common table salt. NaCl is a continuous compound that extends in three-dimensional array much like diamond. NaCl is a solid at room temperature, with a very high melting point (801 °C), similar to the melting points of silver (961.78 °C) and gold (1064.18 °C), although much lower than the decomposition temperature of diamond (3550 °C). An interesting difference between diamond and sodium chloride occurs on heating. Remember diamond does not melt; it decomposes once enough energy is added to the system to break the C–C bonds. Under normal circumstances, the carbon atoms react with oxygen (O2) in the air to form carbon dioxide—a process that requires the addition of lots of energy to reverse (as we will see later). On the other hand NaCl melts (solid → liquid) and freezes (liquid → solid) at 801 °C, much like water, just at a higher temperature. Based on this difference, we might be tempted to conclude that covalent bonds are not broken when salt melts but that something stronger than the H-bonds that hold water molecules together are broken. What could that be?
A hint comes from studies first carried out by the English chemist Humphrey Davy.[1] Davy used a voltaic pile to study the effects of passing electricity through a range of substances.[2] Solid table salt did not conduct electricity, but liquid (molten) salt did. Not only did it conduct electricity, but when electricity (electrons) was passed through it, it decomposed to produce globules of a shiny, highly reactive metal (sodium, Na) and a pale green gas (chlorine, Cl2). Davy correctly (as it turned out) deduced that the elements in table salt (what we now know as sodium and chlorine) are held together by what he termed electrical forces. Just what caused those electrical forces was not discovered until the atomic nature of matter was elucidated over 100 years later.
It takes a great deal of energy to change table salt into its constituent elements. First the salt has to be heated to its melting point, and then electrical energy must be added to release the elements sodium and chlorine. The reverse reaction, combining the elements sodium and chlorine (don’t do this at home), produces sodium chloride and releases a great deal of energy (411 kJ/mol). Given the release of energy, we suspect that bonds are being formed during this reaction.
One of the important principles of chemistry is that structure on the atomic-molecular level is reflected in the behavior of materials in the real world. So, let us review some of the real-world properties of sodium chloride:
- It forms colorless crystals that are often cubical in shape and are hard and brittle.
- It has a high melting point and conducts electricity when melted, but not in the solid state.
Based on these properties, and what we know about interactions, bonds, and electricity, we can begin to make hypotheses about how atoms are organized in NaCl. For example, the fact that NaCl is a stable, crystalline solid at room temperature and that it melts at a high temperature implies that forces holding the atoms together are strong. The regular shape of salt crystals implies that bonds holding the atoms together extend in three dimensions with some regular pattern. If you take a large salt crystal and give it a sharp knock, it breaks cleanly along a flat surface. Diamond also behaves in this way. The ability of molten, but not solid, salt to conduct electricity suggests that melting leads to the appearance of moveable, electrically charged particles. The current interpretation of all these observations and experiments is that in the solid state salt (NaCl) is held together by the coulombic (electrical) attractions between sodium (Na+) and chloride (Cl–) ions. So when sodium metal (Na) reacts with chlorine (Cl2) gas, sodium and chloride ions are produced. In the solid state, these ions are strongly attracted to each other and cannot move, but they can move in the molten (liquid) state, and their movement is what conducts electricity (electrons).
One way to think of ionic bonding is that it is the extreme limit of a polar covalent bond. Typically, simple ionic compounds are formed from elements on the left-hand side of the periodic table (metals, such as sodium) and elements on the right-hand side (non-metals, such as chlorine). The non-metals tend to have a high electronegativity as a result of their high effective nuclear charge, whereas the metals have low electronegativity because their valence electrons are not very strongly attracted to their nuclei. When a metal atom meets a non-metal atom the non-metal attracts the valence electrons from the metal, so that for all intents and purposes electrons move from the metal atom (which then has a net positive charge) to the non-metal atom (which now has a net negative charge). This effect, however, applies only to the electrons in the unfilled valence shells. Electrons in a metal atom’s filled core orbitals require a lot more energy to remove. Why? Because they are closer to the positively charged nucleus (recall the jump in ionization energy when an electron is removed from the core). If there is a single outer-shell electron (as is the case with Na and other Group I metals) that electron is often lost, and the resulting atom (now called an ion) has a single positive charge (for example, Na+). If there are two outer-shell electrons, as in the case of the Group II metals, such as calcium and magnesium, both can be lost to produce doubly charged ions, such as Ca++ and Mg++ (usually written as Ca2+ and Mg2+). At the other side of the periodic table, the non-metals show exactly the opposite pattern, gaining electrons to become negatively charged ions.[3]
Questions
Questions to Answer
- The melting point of table salt is over 800 °C. Why is it so high?
- What properties do you associate with a solid?
- What happens on the atomic-molecular level when a solid melts?
- Why don’t metals tend to gain electrons? Why don’t non-metals lose electrons?
- What happens to the size of a sodium atom when it loses an electron to become Na+?
- What happens to the size of a chlorine atom when it gains an electron and becomes Cl–?
Questions to Ponder
- Why doesn’t solid table salt conduct electricity?
- Why does molten table salt conduct electricity?
Sodium Chloride
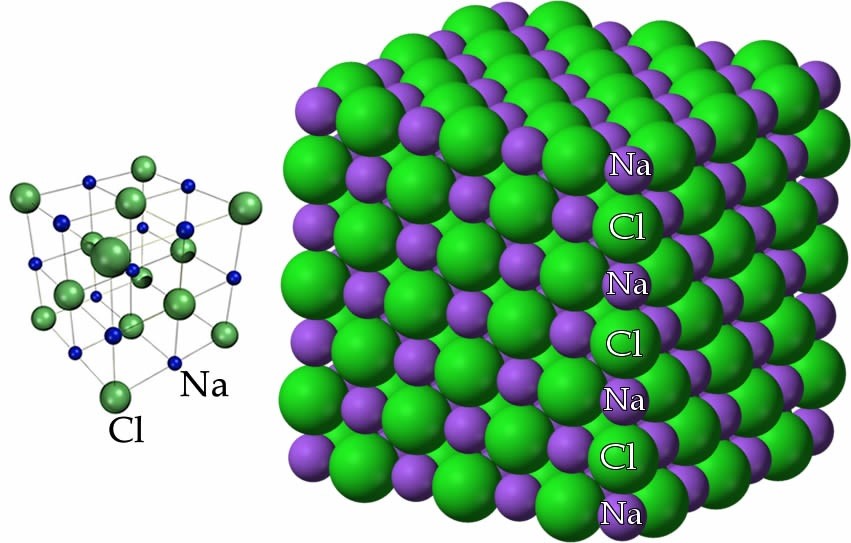
By this point, we have concluded that NaCl is composed of Na+ ions (cations) and Cl– ions (anions), but we have not yet discussed how these ions are arranged with respect to one another in space. As you may have come to expect, there is usually more than one way to represent a chemical structure. Different models emphasize different features of a substance but none of them are real in the sense that if we could look at the molecular-level structure, these models are not what we would see. At the same time, visible cubes of salt crystals provide a clue to atomic-molecular structure. If we follow the structure down from the macroscopic to the molecular, this cubic/rectangular structure is retained. A diagram of sodium chloride showing the relative positions of the ions, shown here, illustrates this cubic organization.
Another way to look at NaCl is to think of each Na+ ion as being surrounded by six Cl– ions, and each Cl– ion is surrounded by six Na+ ions. Such an arrangement is possible because of the relative sizes of the sodium and chloride ions; the smaller Na+ ions can sit in the holes between the larger Cl– ions (why are the chloride ions bigger than the sodium ions?). One consequence of this arrangement is that there is not an “ionic” bond that is analogous to a covalent bond. Our model of bonding here is best understood as this three-dimensional lattice of interacting ions. The alternating network of positive and negative ions makes for a very stable structure that is difficult to disrupt. The implication? Lots of energy is required to break these interactions and allow the ions to move with respect to one another. Many ionic compounds are organized in similar kinds of crystalline structures. A complexity is that many ionic compounds, including NaCl, are highly soluble in water, which means they interact strongly with water molecules. Often salts crystallize together with water molecules and form hydrated (with water) forms, as opposed to anhydrous (without water) forms.
How Ionic Bonding Explains the Properties of Ionic Compounds
Let us return to the properties of ionic compounds and see how this molecular-level (microscopic) model of bonding explains their properties. First, their high melting points arise from the fact that enough energy must be supplied so that multiple (strong) coulombic interactions (recall each cation is surrounded by six anions and vice versa) between the ions must be overcome. In contrast for water, it is only the intermolecular forces between molecules that must be overcome to melt ice; IMFs are significantly weaker than full ionic interactions. Similarly it takes even more energy to vaporize (liquid → gas) NaCl.
Now let us predict the melting points of different ionic compounds. Remember that the force between the ions is a Coulombic attraction:
![]()
where q+ and q– are the charges on the ions, and r is the distance between them. This equation tells us that as the charge on the ions increases, so does the force of attraction, but as the distance between them increases, the force of attraction decreases. That is, the coulombic attraction should be larger for small, highly charged ions, and this should be reflected in the melting points of ionic compounds. Even when we don’t factor in the size of the ions, q1 × q2 = 4 which means that the attractive forces for CaO should be on the order of 4 times those for NaCl. Indeed, the melting point of calcium oxide (CaO) which has q1 = 2+ and q2 = 2– is 2,572 °C.
Questions
Questions to Answer
- Draw a molecular level picture of liquid water, and a molecular level picture of liquid sodium chloride. Use this picture to explain why it takes more energy to melt solid salt than it does to melt solid water.
- Arrange these ionic compounds in order of increasing melting point: NaCl, KBr, CaO, Al2O3. Look up your answers and see if your predictions were correct.
- Arrange these materials in order of increasing melting point: CH4, MgBr2, HF, C(diamond). Look up your answers and see if your predictions were correct.
- What do you think happens to the size of the particle when a chlorine atom gains an electron to become a chloride ion? (hint recall that the size of an atom depends on the balance between the attractions between the electrons and the nucleus, and the repulsions between the electrons)
- What do you think happens to the size of the particle when a sodium atom loses an electron to become a sodium ion?
- While Davy is well known now for his experiments on the nature of salts, he began his chemical career in his early twenties researching medical uses of gases. He apparently became very fond of nitrous oxide (N2O, laughing gas), which he reported was an enjoyable recreational drug and a cure for hangovers (ref SALT). ↵
- In 1800 the first electric battery, the Voltaic Pile, was developed. It was promptly put to use by a growing number of scientists. For example, molecular hydrogen and oxygen could be produced by passing electricity through water. ↵
- Positively charged ions are known as cations and negatively charged ions are known as anions. ↵

