Monoatomic and Polyatomic Ions
Predictable Charges
You can often use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group (s and p block) metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. In other words, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; an alkaline earth metal (group 2) loses two electrons and forms a cation with a 2+ charge, and so on. For example, a neutral calcium atom, with 20 protons and 20 electrons, loses two electrons. This results in a cation with 20 protons, 18 electrons, and a 2+ charge. It is symbolized Ca2+, and has the same number of electrons as atoms of the preceding noble gas, argon. The name of a metal ion is the same as the name of the metal atom from which it forms, so Ca2+ is called a calcium ion.
When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. Atoms of group 17 gain one electron and form anions with a 1− charge; atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron. This results in an anion with 35 protons, 36 electrons, and a 1− charge. It is symbolized Br−, and has the same number of electrons as atoms of the next noble gas, krypton.
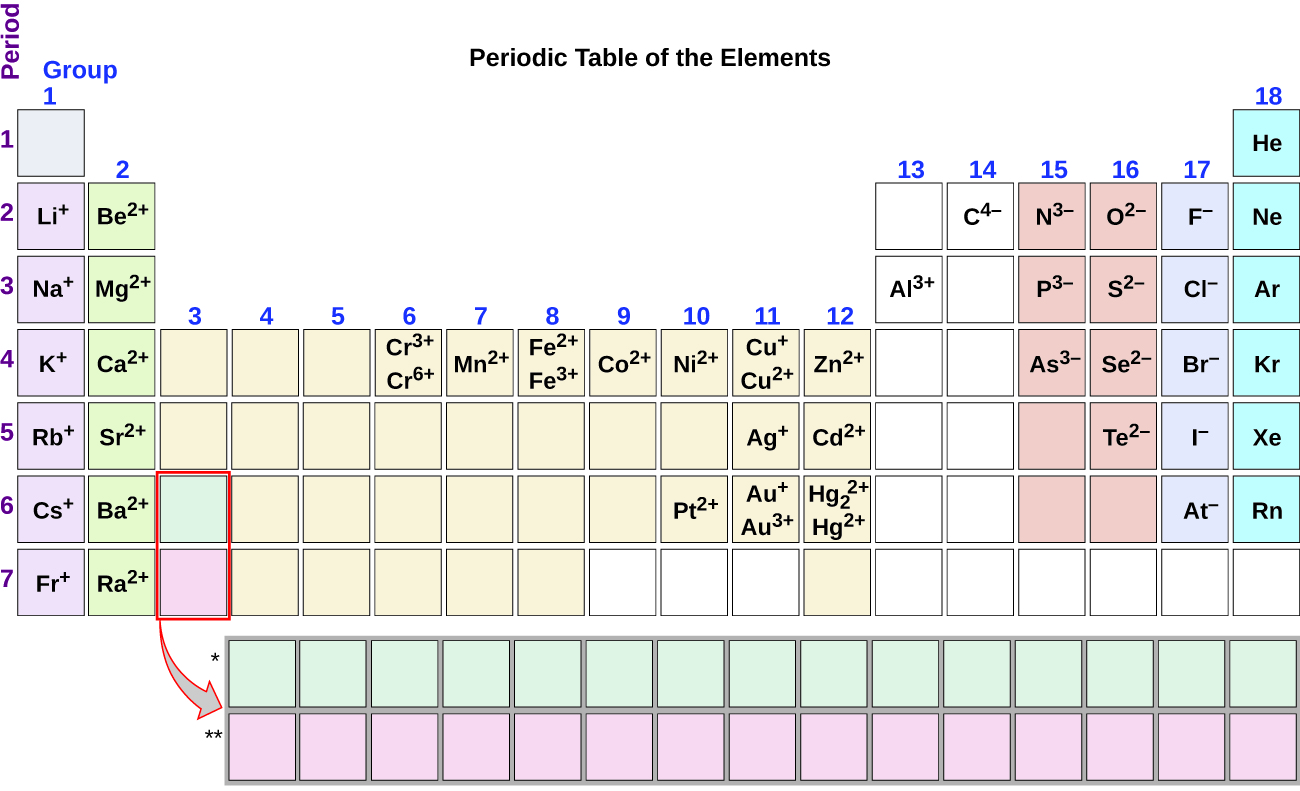
Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on. Moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. For example, group 17 elements (one group left of the noble gases) form 1− ions; group 16 elements (two groups left) form 2− ions, and so on. This trend can be used as a guide in many cases, but its predictive value decreases when moving toward the center of the periodic table. In fact, transition metals and some other metals often exhibit variable charges that are not predictable by their location in the table. For example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge.
Example 1
Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
Solution
Magnesium’s position in the periodic table (group 2) tells us that it is a metal. Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is Mg2+, and it is called a magnesium ion.
Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon. Thus, a nitrogen atom will form an anion with three more electrons than protons and a charge of 3−. The symbol for the ion is N3−, and it is called a nitride ion.
Check Your Learning
Aluminum and carbon react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
Answer
Al will form a cation with a charge of 3+: Al3+, an aluminum ion. Carbon will form an anion with a charge of 4−: C4−, a carbide ion.
Example 2
The gemstone sapphire is mostly a compound of aluminum and oxygen that contains aluminum cations, Al3+, and oxygen anions, O2−. What is the formula of this compound?
Solution
Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative charges. The formula would be Al2O3.
Check Your Learning
Predict the formula of the ionic compound formed between the sodium cation, Na+, and the sulfide anion, S2−.
Answer
Na2S
Because an ionic compound is not made up of discrete molecules, it may not be properly symbolized using a molecular formula. Instead, ionic compounds are symbolized by a formula indicating the relative numbers of its constituent ions, using the simplest whole-number ratios. For compounds containing only monatomic ions (such as NaCl), these formulas are just the empirical formulas.
Naming Ionic Compounds
Compounds Containing Only Monatomic Ions
The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix –ide). Some examples are given below.
| NaCl, sodium chloride | Na2O, sodium oxide |
| KBr, potassium bromide | CdS, cadmium sulfide |
| CaI2, calcium iodide | Mg3N2, magnesium nitride |
| CsF, cesium fluoride | Ca3P2, calcium phosphide |
| LiCl, lithium chloride | Al4C3, aluminum carbide |
Compounds Containing a Metal Ion with a Variable Charge
Most of the transition metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as above, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ (Figure 1), and the two corresponding compound formulas are FeCl2 and FeCl3. The simplest name, “iron chloride,” will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Other examples are provided below.
| Transition Metal Ionic Compound | Name |
| FeCl3 | iron(III) chloride |
| FeCl2 | iron(II) chloride |
| Hg2O | mercury(I) oxide |
| HgO | mercury(II) oxide |
Out-of-date nomenclature used the suffixes –ic and –ous to designate metals with higher and lower charges, respectively: Iron(III) chloride, FeCl3, was previously called ferric chloride, and iron(II) chloride, FeCl2, was known as ferrous chloride. Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. For example, you may see the words stannous fluoride on a tube of toothpaste. This represents the formula SnF2, which is more properly named tin(II) fluoride. The other fluoride of tin is SnF4, which was previously called stannic fluoride but is now named tin(IV) fluoride.
Example 3
Name the following ionic compounds, which contain a metal that can have more than one ionic charge:
- Fe2S3
- CuSe
- GaN
- CrCl3
Solution
The anions in these compounds have a fixed negative charge (S2−, Se2− , N3−, and Cl−), and the compounds must be neutral. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, and Cr3+. These charges are used in the names of the metal ions:
- iron(III) sulfide
- copper(II) selenide
- gallium(III) nitride
- chromium(III) chloride
Check Your Learning
Write the formulas of the following ionic compounds:
- chromium(III) phosphide
- mercury(II) sulfide
- manganese(II) oxide
- copper(I) oxide
Answer
(a) CrP; (b) HgS; (c) MnO; (d) Cu2O
Polyatomic Ions
The ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one atom. We also find many polyatomic ions. These ions, which act as discrete units, are electrically charged molecules (a group of covalently-bonded atoms with an overall charge). Oxyanions are polyatomic ions that contain one or more oxygen atoms. Table below lists some of the common polyatomic ions.
| Polyatomic Ion Name | Chemical Formula | Polyatomic Ion Name | Chemical Formula |
| ammonium | NH4+ | hydrogen carbonate | HCO3– |
| hydronium | H3O+ | carbonate | CO32- |
| hydroxide | OH– | hydrogen sulfate | HSO4– |
| acetate | CH3COO– | sulfate | SO42- |
| nitrate | NO3– | phosphate | PO43- |
| permanganate | MnO4– |
| Name | Formula | Name | Formula |
| oxide | O2- | hydrogen phosphate | HPO42- |
| peroxide | O22- | dihydrogen phosphate | H2PO4– |
| cyanide | CN– | perchlorate | ClO4– |
| azide | N3– | chlorate | ClO3– |
| nitrite | NO2– | chlorite | ClO2– |
| hydrogen sulfite | HSO3– | hypochlorite | ClO– |
| sulfite | SO32- | chromate | CrO42- |
| dichromate | Cr2O72- |
Note that there is a system for naming some polyatomic ions; -ate and -ite are suffixes designating polyatomic ions containing more or fewer oxygen atoms. Per- (short for “hyper” meaning “above”) and hypo- (meaning “under”) are prefixes meaning more oxygen atoms than -ate and fewer oxygen atoms than -ite, respectively. For example, perchlorate is ClO4−, chlorate is ClO3−, chlorite is ClO2− and hypochlorite is ClO−. Unfortunately, the number of oxygen atoms corresponding to a given suffix or prefix is not consistent; for example, nitrate is NO3− while sulfate is SO42−.
Naming Polyatomic Ions
Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an –ide ending, since the suffix is already present in the name of the anion. Examples are shown below.
| KC2H3O2, potassium acetate | NH4Cl, ammonium chloride |
| NaHCO3, sodium hydrogen carbonate | CaSO4, calcium sulfate |
| Al2(CO3)3, aluminum carbonate | Mg3(PO4)2, magnesium phosphate |
Many ionic compounds contain polyatomic ions as the cation, the anion, or both. As with simple ionic compounds, these compounds must also be electrically neutral, so their formulas can be predicted by treating the polyatomic ions as discrete units. We use parentheses in a formula to indicate a group of atoms that behave as a unit. For example, the formula for calcium phosphate, one of the minerals in our bones, is Ca3(PO4)2. This formula indicates that there are three calcium ions (Ca2+) for every two phosphate (PO43−) groups. The PO43− groups are discrete units, each consisting of one phosphorus atom and four oxygen atoms, and having an overall charge of 3−. The compound is electrically neutral, and its formula shows a total count of three Ca, two P, and eight O atoms.
Ionic Compounds in Your Cabinets
Every day you encounter and use a large number of ionic compounds. Some of these compounds, where they are found, and what they are used for are listed in Table below. Look at the label or ingredients list on the various products that you use during the next few days, and see if you run into any of those in this table, or find other ionic compounds that you could now name or write as a formula.
| Ionic Compound | Use |
|---|---|
| NaCl, sodium chloride | ordinary table salt |
| KI, potassium iodide | added to “iodized” salt for thyroid health |
| NaF, sodium fluoride | ingredient in toothpaste |
| NaHCO3, sodium hydrogen carbonate (aka sodium bicarbonate) | baking soda; used in cooking (and as antacid) |
| Na2CO3, sodium carbonate | washing soda; used in cleaning agents |
| NaOCl, sodium hypochlorite | active ingredient in household bleach |
| CaCO3 calcium carbonate | ingredient in antacids |
| Mg(OH)2, magnesium hydroxide | ingredient in antacids |
| Al(OH)3, aluminum hydroxide | ingredient in antacids |
| NaOH, sodium hydroxide | lye; used as drain cleaner |
| K3PO4, potassium phosphate | food additive (many purposes) |
| MgSO4, magnesium sulfate | added to purified water |
| Na2HPO4, sodium hydrogen phosphate | anti-caking agent; used in powdered products |
| Na2SO3, sodium sulfite | preservative |
Example 4
Baking powder contains calcium dihydrogen phosphate, an ionic compound composed of the ions Ca2+ and H2PO4−. What is the formula of this compound?
Solution
The positive and negative charges must balance, and this ionic compound must be electrically neutral. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. This requires a ratio of one Ca2+ ion to two H2PO4− ions. We designate this by enclosing the formula for the dihydrogen phosphate ion in parentheses and adding a subscript 2. The formula is Ca(H2PO4)2.
Check Your Learning
Predict the formula of the ionic compound formed between the lithium ion and the peroxide ion, O22− (Hint: Use the periodic table to predict the sign and the charge on the lithium ion.)
Answer
Li2O2
Because an ionic compound is not made up of single, discrete molecules, it may not be properly symbolized using a molecular formula. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. For compounds containing only monatomic ions (such as NaCl) and for many compounds containing polyatomic ions (such as CaSO4), these formulas are just the empirical formulas. However, the formulas for some ionic compounds containing polyatomic ions are not empirical formulas. For example, the ionic compound sodium oxalate is comprised of Na+ and C2O42− ions combined in a 2:1 ratio, and its formula is written as Na2C2O4. The subscripts in this formula are not the smallest-possible whole numbers, as each can be divided by 2 to yield the empirical formula, NaCO2. This, however, is not the accepted formula for sodium oxalate, as it does not accurately represent the compound’s polyatomic anion, C2O42−.
Lattice Energy
The lattice energy of an ionic compound is a measure of the strength of the electrostatic attraction between its positive and negative ions. The lattice energy (ΔHlattice) of an ionic compound is defined as the energy required to separate one mole of the solid into its component gaseous ions. For the ionic solid, MX, the lattice energy is the enthalpy change of the process:
MX(s) → Mn+(g) + Xn-(g) ΔHlattice
Note that we are using the convention where the ionic solid is separated into ions, so our lattice energies will be endothermic (positive values). Some texts use the equivalent but opposite convention, defining lattice energy as the energy released when separate ions combine to form a lattice and giving negative (exothermic) values. Thus, if you are looking up lattice energies in another reference, be certain to check which definition is being used. In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. For sodium chloride, ΔHlattice = 769 kJ/mol. Thus, it requires 769 kJ to separate one mole of solid NaCl into gaseous Na+ and Cl– ions. When one mole each of gaseous Na+ and Cl– ions form solid NaCl, 769 kJ of heat is released.
The lattice energy ΔHlattice of an ionic crystal can be expressed by the following equation (derived from Coulomb’s law, governing the forces between electric charges):
ΔHlattice = ![]()
in which C is a constant that depends on the type of crystal structure; Z+ and Z– are the charges on the ions; and Ro is the interionic distance (the sum of the radii of the positive and negative ions). Thus, the lattice energy of an ionic crystal increases rapidly as the charges of the ions increase and the sizes of the ions decrease. When all other parameters are kept constant, doubling the charge of both the cation and anion quadruples the lattice energy. For example, the lattice energy of LiF (Z+ and Z– = 1) is 1023 kJ/mol, whereas that of MgO (Z+ and Z– = 2) is 3900 kJ/mol (Ro is nearly the same—about 200 pm for both compounds).
Different interatomic distances produce different lattice energies. For example, we can compare the lattice energy of MgF2 (2957 kJ/mol) to that of MgI2 (2327 kJ/mol) to observe the effect on lattice energy of the smaller ionic size of F– as compared to I–.
Example 5
The precious gem ruby is aluminum oxide, Al2O3, containing traces of Cr3+. The compound Al2Se3 is used in the fabrication of some semiconductor devices. Which has the larger lattice energy, Al2O3 or Al2Se3?
Solution
In these two ionic compounds, the charges Z+ and Z– are the same, so the difference in lattice energy will depend upon R0. The O2– ion is smaller than the Se2– ion. Thus, Al2O3 would have a shorter interionic distance than Al2Se3, and Al2O3 would have the larger lattice energy.
Check Your Learning
Zinc oxide, ZnO, is a very effective sunscreen. How would the lattice energy of ZnO compare to that of NaCl?
Answer
ZnO would have the larger lattice energy because the Z values of both the cation and the anion in ZnO are greater, and the interionic distance of ZnO is smaller than that of NaCl.

