Elements and Isotopes
The Structure of the Atom
Much of our current understanding of atoms and atomic structure was developed in the early part of the 20th century. The work of Thomson, Millikan, and others revealed the charge and mass of the negative subatomic particles—the electrons. We designate electrons with the symbol “e–“, the superscript negative sign emphasizes their negative charge. The surprising results of Rutherford’s experiments indicated that the positively charged subatomic particles—the protons (p+)—are much more massive than electrons (by a factor of about 2,000) and are highly concentrated in the atom’s nucleus, which occupies just a tiny portion of the atom’s entire volume. A third subatomic component that bears no electrical charge—the neutrons (n0)—have masses similar to protons and co-locate with protons in the nucleus.
For a perspective about their relative sizes, consider this: if the nucleus were the size of a blueberry, the atom would be about the size of a football stadium (Figure 1).


Most of the volume of an atom is occupied by the “cloud” of electrons. We use the term “electron cloud” because the exact positions of the electrons cannot be known, we simply know that they are distributed about the volume of the atom (Figure 2).
Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 × 10−23 g, and an electron has a charge of less than 2 × 10−19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). The amu was originally defined based on hydrogen, the lightest element, then later in terms of oxygen. Since 1961, it has been defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. (This isotope is known as “carbon-12”.) Thus, one amu is exactly ![]() of the mass of one carbon-12 atom. Moreover, 1 amu = 1.6605 × 10−24 g. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C.
of the mass of one carbon-12 atom. Moreover, 1 amu = 1.6605 × 10−24 g. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C.
A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of 0.0005486 amu (it would take 1836 electrons to equal the mass of one proton). The properties of these fundamental particles are summarized in Table 1. (An observant student might notice that the sum of an atom’s subatomic particles does not equal the atom’s actual mass: The total mass of six protons, six neutrons, and six electrons is 12.099 amu, slightly larger than 12.00 amu. This “missing” mass is known as the mass defect, which is beyond the scope of this course.)
| Name | Location | Charge (C) | Unit Charge | Mass (amu) | Mass (g) |
| electron | outside nucleus | −1.602 × 10−19 | 1− | 0.0005486 | 0.0009109 × 10−28 |
| proton | nucleus | 1.602 × 10−19 | 1+ | 1.00728 | 1.67262 × 10−24 |
| neutron | nucleus | 0 | 0 | 1.00866 | 1.67493 × 10−24 |
Example 1: Mass Interconversion
If a person’s mass is 100 kg, what is their mass in amu’s?
Solution
1 amu = 1.6605 × 10−24 g. Therefore, the person’s mass is given by:
100 kg × ![]() ×
× ![]() = 6.022 × 1028 amu
= 6.022 × 1028 amu
Check Your Learning
What is the mass in grams of a proton that has mass of 1.0073 amu?
Answer
1.6726 × 10-24 g
The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element: its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons in a neutral atom. Therefore, the atomic number also indicates the number of electrons in an atom. The total number of protons and neutrons in an atom is called its mass number (A). The number of neutrons is therefore the difference between the mass number and the atomic number: A – Z = number of neutrons.
atomic number (Z) = number of protons
mass number (A) = number of protons + number of neutrons
A – Z = number of neutrons
Isotopes of an element are atoms with the same atomic number but different mass numbers; isotopes of an element, therefore, differ from each other only in the number of neutrons within the nucleus.
Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. The charge of an atom is defined as:
Atomic charge = number of protons − number of electrons
Atoms (and molecules) typically acquire charge by gaining or losing electrons. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). A neutral oxygen atom (Z = 8) has eight electrons, and if it gains two electrons it will become an anion with a 2- charge (8 − 10 = 2-).
Example 2: Composition of an Atom
Iodine is an essential trace element in our diet; it is needed to produce thyroid hormone. Insufficient iodine in the diet can lead to the development of a goiter, an enlargement of the thyroid gland (Figure 3).
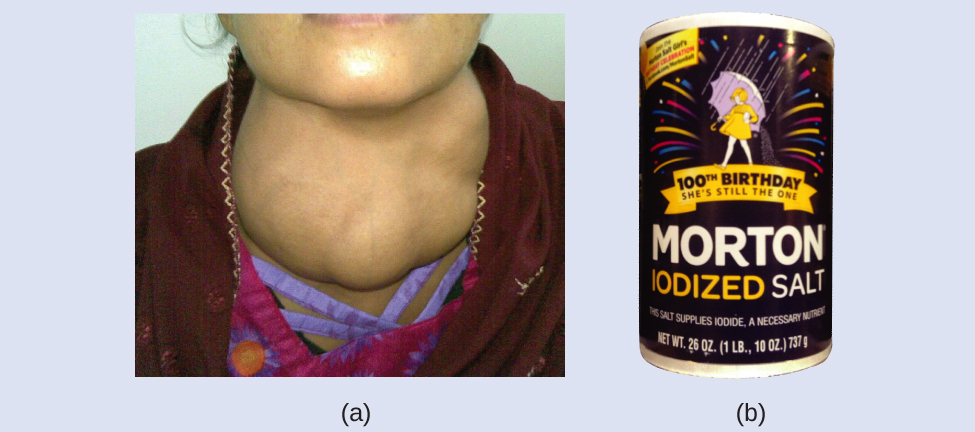
The addition of small amounts of iodine to table salt (iodized salt) has essentially eliminated this health concern in the United States, but as much as 40% of the world’s population is still at risk of iodine deficiency. The iodine atoms are added as anions, and each has a 1− charge and a mass number of 127. Determine the numbers of protons, neutrons, and electrons in one of these iodine anions.
Solution
The atomic number of iodine (53) tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 – 53 = 74). Since the iodine is added as a 1- anion, the number of electrons is 54 [53 – (1–) = 54].
Check Your Learning
An ion of platinum has a mass number of 195 and contains 74 electrons. How many protons and neutrons does it contain, and what is its charge?
Answer
78 protons; 117 neutrons; charge is 4+
Chemical Symbols
A chemical symbol is an abbreviation that we use to indicate an element or an atom of an element. For example, the symbol for mercury is Hg (Figure 4). We use the same symbol to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).

No doubt you are familiar with many elements and their symbols already, the names and symbols for elements 1 through 36 as well as some other commonly encountered elements are listed in Table 2. Some symbols are derived from the common name of the element; others are abbreviations of the name in another language. Most symbols have one or two letters, but three-letter symbols have been used to describe some elements that have atomic numbers greater than 112. To avoid confusion with other notations, only the first letter of a symbol is capitalized. For example, Co is the symbol for the element cobalt, but CO is the notation for the compound carbon monoxide, which contains atoms of the elements carbon (C) and oxygen (O).
| Element | Symbol | Element | Symbol | Element | Symbol |
| hydrogen | H | sulfur | S | gallium | Ga |
| helium | He | chlorine | Cl | germanium | Ge |
| lithium | Li | argon | Ar | arsenic | As |
| beryllium | Be | potassium | K | selenium | Se |
| boron | B | calcium | Ca | bromine | Br |
| carbon | C | scandium | Sc | krypton | Kr |
| nitrogen | N | titanium | Ti | ||
| oxygen | O | vanadium | V | ||
| fluorine | F | chromium | Cr | silver | Ag |
| neon | Ne | manganese | Mn | gold | Au |
| sodium | Na | iron | Fe | barium | Ba |
| magnesium | Mg | cobalt | Co | iodine | I |
| aluminum | Al | nickel | Ni | mercury | Hg |
| silicon | Si | copper | Cu | lead | Pb |
| phosphorus | P | zinc | Zn | uranium | U |
Traditionally, the discoverer (or discoverers) of a new element names the element. However, until the name is recognized by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of the new element is based on the Latin word(s) for its atomic number. For example, element 106 was called unnilhexium (Unh), element 107 was called unnilseptium (Uns), and element 108 was called unniloctium (Uno) for several years. These elements are now named after scientists (or occasionally locations); for example, element 106 is now known as seaborgium (Sg) in honor of Glenn Seaborg, a Nobel Prize winner who was active in the discovery of several heavy elements.
Isotopes
The symbol for a specific isotope of any element is written by placing the mass number as a superscript to the left of the element symbol (Figure 5).
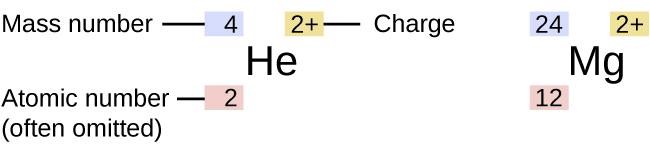
The atomic number is sometimes written as a subscript preceding the symbol, but since this number defines the element’s identity, as does its symbol, it is often omitted. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24Mg, 25Mg, and 26Mg. These isotope symbols are read as “element, mass number” and can be symbolized consistent with this reading. For instance, 24Mg is read as “magnesium 24,” and can be written as “magnesium-24” or “Mg-24.” 25Mg is read as “magnesium 25,” and can be written as “magnesium-25” or “Mg-25.” All magnesium atoms have 12 protons in their nucleus. They differ only because a 24Mg atom has 12 neutrons in its nucleus, a 25Mg atom has 13 neutrons, and a 26Mg has 14 neutrons.
Information about the naturally occurring isotopes of elements with atomic numbers 1 through 10 is given in Table 3. Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolized 2H, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized 3H, is also called tritium and sometimes symbolized T.
| Element | Symbol | Atomic Number | Number of Protons | Number of Neutrons | Mass (amu) | % Natural Abundance |
| hydrogen | 1 | 1 | 0 | 1.0078 | 99.989 | |
| 1 | 1 | 1 | 2.0141 | 0.0115 | ||
| 1 | 1 | 2 | 3.01605 | — (trace) | ||
| helium | 2 | 2 | 1 | 3.01603 | 0.00013 | |
| 2 | 2 | 2 | 4.0026 | 100 | ||
| lithium | 3 | 3 | 3 | 6.0151 | 7.59 | |
| 3 | 3 | 4 | 7.0160 | 92.41 | ||
| beryllium | 4 | 4 | 5 | 9.0122 | 100 | |
| boron | 5 | 5 | 5 | 10.0129 | 19.9 | |
| 5 | 5 | 6 | 11.0093 | 80.1 | ||
| carbon | 6 | 6 | 6 | 12.0000 | 98.89 | |
| 6 | 6 | 7 | 13.0034 | 1.11 | ||
| 6 | 6 | 8 | 14.0032 | — (trace) | ||
| nitrogen | 7 | 7 | 7 | 14.0031 | 99.63 | |
| 7 | 7 | 8 | 15.0001 | 0.37 | ||
| oxygen | 8 | 8 | 8 | 15.9949 | 99.757 | |
| 8 | 8 | 9 | 16.9991 | 0.038 | ||
| 8 | 8 | 10 | 17.9992 | 0.205 | ||
| fluorine | 9 | 9 | 10 | 18.9984 | 100 | |
| neon | 10 | 10 | 10 | 19.9924 | 90.48 | |
| 10 | 10 | 11 | 20.9938 | 0.27 | ||
| 10 | 10 | 12 | 21.9914 | 9.25 |
Demonstration: Heavy water is more dense than regular water
Set up. As shown in the table above, hydrogen and oxygen each have three isotopes. Of these isotopes, hydrogen-1 and oxygen-16 are by far the most abundant isotopes. Therefore, water molecules typically consist of two hydrogen-1 atoms and one oxygen-16 atom, giving a molecular weight of 18 amu. It is possible to form “heavy water”—water that is composed of two hydrogen-2 atoms and one oxygen-16 atom. This heavy water has a molecular weight of 20 amu. In this demonstration, we compare the density of solid regular water (regular ice) and solid heavy water (“heavy water ice”) by placing each into a glass of liquid regular water.
Prediction. Before watching the video, make a prediction about whether the heavy water ice will float or sink in the glass of liquid water.
Explanation. In this video, the regular ice floats on the liquid regular water, as expected for an ice cube in a glass of water because the density of solid (regular) water is less than the density of liquid (regular) water. However, the heavy water ice cube sinks to the bottom of the glass because the extra mass causes the density of the heavy water ice to be greater than the density of the liquid regular water, and therefore it does not float.
Natural Abundance
Because each proton and each neutron contributes 1 amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single atom is approximately equal to its mass number (a whole number). However, the average masses of atoms of most elements are not whole numbers because most elements exist naturally as mixtures of two or more isotopes.
The mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted average mass of all the isotopes present in a naturally occurring sample of that element. This is equal to the sum of each individual isotope’s mass multiplied by its fractional abundance.
average mass = ∑i (fractional abundance × isotopic mass)i
For example, the element boron is composed of two isotopes: 19.9% of all boron atoms are 10B with a mass of 10.0129 amu, and the remaining 80.1% are 11B with a mass of 11.0093 amu. The average atomic mass for boron is calculated to be:
| boron average mass | = | (0.199 × 10.0129 amu) + (0.801 × 11.0093 amu) |
| = | 1.99 amu + 8.82 amu | |
| = | 10.81 amu |
It is important to understand that no single boron atom weighs exactly 10.8 amu—10.8 amu is the average mass of all boron atoms, and individual boron atoms weigh either 10 amu or 11 amu.
Example 3: Calculation of Average Atomic Mass
A meteorite found in central Indiana contains traces of the noble gas neon picked up from the solar wind during the meteorite’s trip through the solar system. Analysis of a sample of the gas showed that it consisted of 91.84% 20Ne (mass 19.9924 amu), 0.47% 21Ne (mass 20.9940 amu), and 7.69% 22Ne (mass 21.9914 amu). What is the average mass of the neon in the solar wind?
Solution
| neon average mass | = | (0.9184 × 19.9924 amu) + (0.0047 × 20.9940 amu) + (0.0769 × 21.9914 amu) |
| = | (18.36 + 0.099 + 1.69) amu | |
| = | 20.15 amu |
The average mass of neon in the solar wind is 20.15 amu. (The average mass of a terrestrial neon atom is 20.1796 amu. This result demonstrates that we may find slight differences in the natural abundance of isotopes depending on their origin.)
Check Your Learning
A sample of magnesium is found to contain 78.70% of 24Mg atoms (mass 23.98 amu), 10.13% of 25Mg atoms (mass 24.99 amu), and 11.17% of 26Mg atoms (mass 25.98 amu). Calculate the average mass of Mg.
Answer
24.31 amu
We can also do variations of this type of calculation, as shown in the next example.
Example 4: Calculation of Percent Abundance
Naturally occurring chlorine consists of 35Cl (mass 34.96885 amu) and 37Cl (mass 36.96590 amu), with an average mass of 35.453 amu. What is the percent composition of Cl in terms of these two isotopes?
Solution
The average mass of chlorine is the fraction that is 35Cl times the mass of 35Cl plus the fraction that is 37Cl times the mass of 37Cl.
chlorine average mass = (fraction of 35Cl × mass of 35Cl) + (fraction of 37Cl × mass of 37Cl)
If we let x represent the fraction that is 35Cl, then the fraction that is 37Cl is represented by 1.00 − x. (The fraction that is 35Cl + the fraction that is 37Cl must add up to 1, so the fraction of 37Cl must equal 1.00 − the fraction of 35Cl.)
Substituting this into the average mass equation, we have:
| 35.453 amu | = | (x × 34.96885 amu) + [(1.00 – x) × 36.96590 amu] |
| 35.453 | = | 34.96885x + 36.96590 – 36.96590x |
| 1.99705x | = | 1.513 |
| x | = | |
| x | = | 0.7576 |
Therefore, x = 0.7576, which means that 1.00 − 0.7576 = 0.2424. The result is that chlorine consists of 75.76% 35Cl and 24.24% 37Cl.
Check Your Learning
Naturally occurring copper consists of 63Cu (mass 62.9296 amu) and 65Cu (mass 64.9278 amu), with an average mass of 63.546 amu. What is the percent composition of Cu in terms of these two isotopes?
Answer
69.15% Cu-63 and 30.85% Cu-65

