VSEPR Model
To understand molecular behavior, it is important to be able to describe bonds in terms of their distances, angles, and relative arrangements in space (Figure 1).

A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are typically measured in Ångstroms (1 Å = 10–10 m) or picometers (1 pm = 10–12 m, 100 pm = 1 Å).
Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular geometry, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them. The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions as far from each other as possible.
VSEPR theory predicts the arrangement of electron pairs around each central atom and, usually, the correct arrangement of atoms in a molecule using only electron pair repulsions. This neglects other important factors which contribute to electronic and molecular geometries such as orbital mixing, π conjugation, hyperconjugation, dipole-dipole interactions, hydrogen-bonding, etc. Many of these other factors will be explored in this course and future chemistry courses.
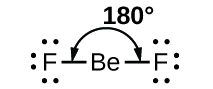 As a simple example of VSEPR theory, let us predict the structure of a gaseous BeF2 molecule. The Lewis structure of BeF2 shows only two electron pairs around the central beryllium atom. With two bonds and no lone pairs of electrons on the central atom, the bonds are as far apart as possible, and the electrostatic repulsion between these regions of high electron density is reduced to a minimum when they are on opposite sides of the central atom. The F-Be-F bond angle is 180° in the BeF2 molecule resulting in a linear geometry.
As a simple example of VSEPR theory, let us predict the structure of a gaseous BeF2 molecule. The Lewis structure of BeF2 shows only two electron pairs around the central beryllium atom. With two bonds and no lone pairs of electrons on the central atom, the bonds are as far apart as possible, and the electrostatic repulsion between these regions of high electron density is reduced to a minimum when they are on opposite sides of the central atom. The F-Be-F bond angle is 180° in the BeF2 molecule resulting in a linear geometry.
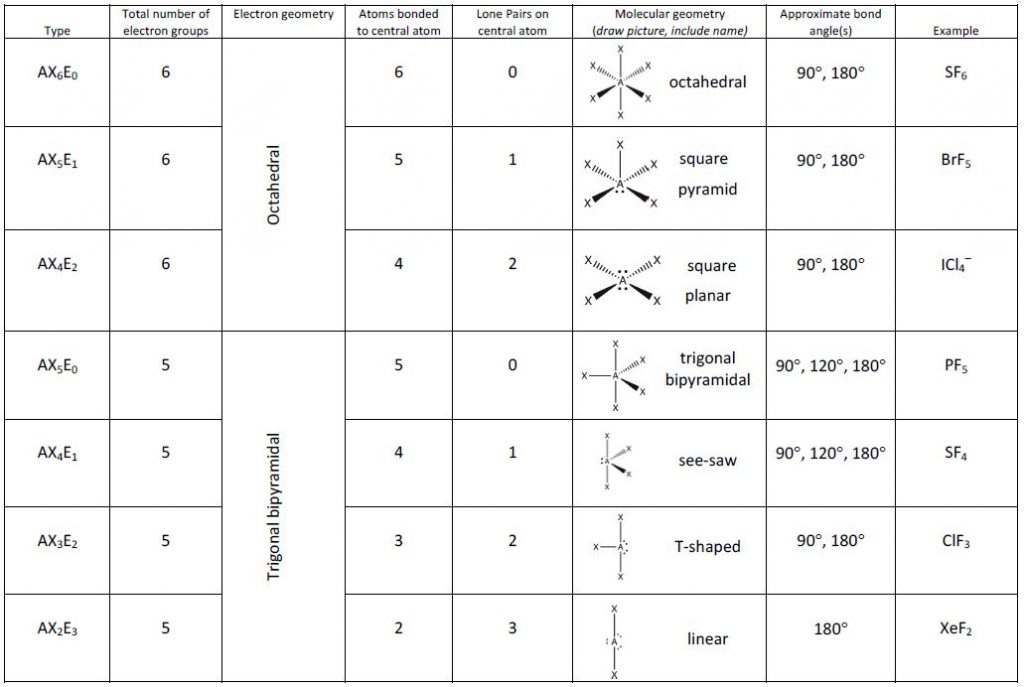
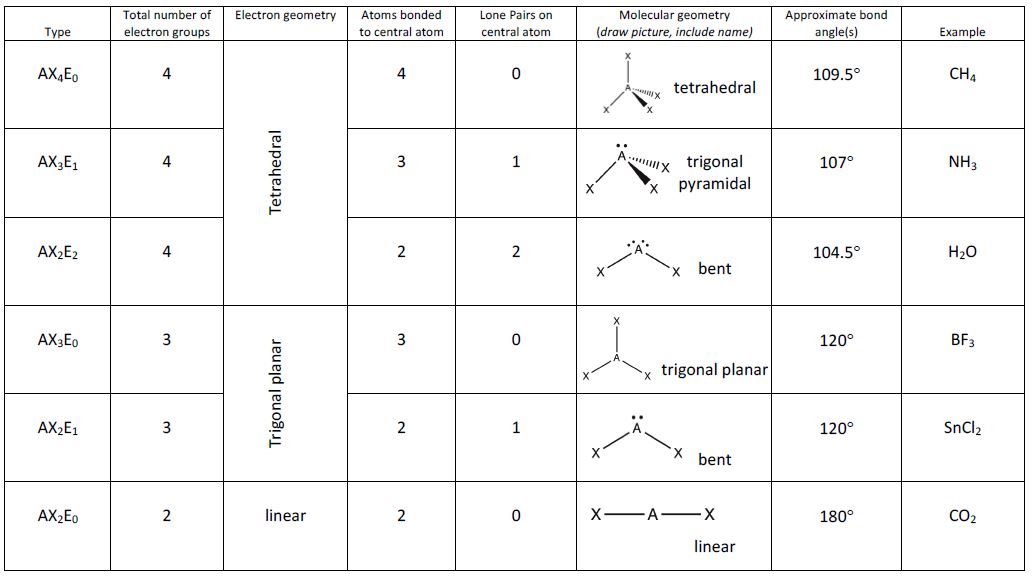
Figure 2 illustrates this and other electron-pair geometries that minimize the repulsions among regions of high electron density (bonds and/or lone pairs). Two regions of electron density around a central atom in a molecule form a linear geometry; three regions form a trigonal planar geometry; four regions form a tetrahedral geometry; five regions form a trigonal bipyramidal geometry; and six regions form an octahedral geometry.

Electron-pair Geometry vs. Molecular Geometry
It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular geometry. The electron-pair geometries shown in Figure 2 describe all regions where electrons are located, bonds as well as lone pairs. Molecular geometry describes the location of the atoms, not the lone pairs. Even though molecular geometry does not describe the location of the lone pairs, the lone pairs still influence the location of the atoms and this influence is still taken into account.
We differentiate between these two situations by naming the geometry that includes all electron pairs the electron-pair geometry. The structure that includes only the placement of the atoms in the molecule is called the molecular geometry. The electron-pair geometries will be the same as the molecular geometry when there are no lone electron pairs around the central atom, but they will be different when there are lone pairs present on the central atom.
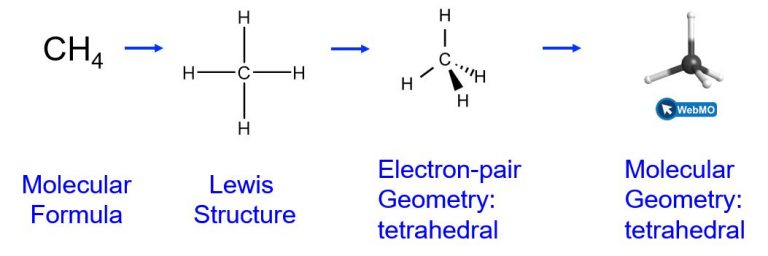
For example, the methane molecule, CH4, which is the major component of natural gas, has four bonding pairs of electrons (i.e., four regions of electron density) around the central carbon atom; the electron-pair geometry is tetrahedral, as is the molecular geometry.
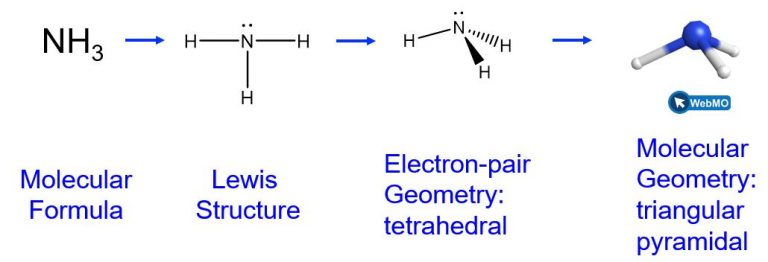
The ammonia molecule, NH3, also has four electron pairs associated with the nitrogen atom (i.e., four regions of electron density), and thus has a tetrahedral electron-pair geometry. One of these regions, however, is a lone pair, which is not included in the molecular geometry, though it does influence the shape of the molecule.
In the ammonia molecule, the three hydrogen atoms attached to the central nitrogen are not arranged in a flat, trigonal planar molecular geometry, but rather in a three-dimensional trigonal pyramid with the nitrogen atom at the apex and the three hydrogen atoms forming the base. The ideal bond angles in a trigonal pyramid are based on the tetrahedral electron pair geometry. Small bond angle distortions occur due to the lone electron pair on nitrogen. The H–N–H bond angles in NH3 are slightly smaller than the 109.5° angle in a regular tetrahedron.
Figure 3 illustrates the ideal molecular geometries, which are predicted based on the electron-pair geometries for various combinations of lone pairs and bonding pairs. Because wedge and dash structures are often confusing at first, we also provide a typical representation of a wedge and dash structure for each combination. While there are other ways that you could rotate the molecule in space and different placements of the wedges and dashes that would also be considered correct, these depictions have been chosen to be the most straightforward to draw and interpret.
Example 1: Predicting Electron-pair Geometry and Molecular Geometry: Lone Pairs on the Central Atom
Predict the electron-pair geometry and molecular geometry of a water molecule.
Solution
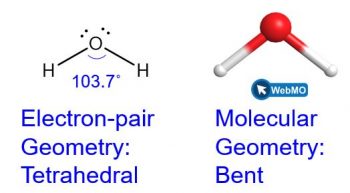 The Lewis structure of H2O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two chemical bonds:
The Lewis structure of H2O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two chemical bonds:
We predict that these four regions are arranged in a tetrahedral (Figure 3) fashion to minimize the electron repulsion, as shown. Thus, the electron-pair geometry is tetrahedral and the molecular geometry is bent with an angle slightly less than 109.5°. In fact, the bond angle is predicted to be 103.7°.
Check Your Learning
The hydronium ion, H3O+, forms when acids are dissolved in water. Predict the electron-pair geometry and molecular geometry of this cation.
Answer
electron pair geometry: tetrahedral; molecular geometry: trigonal pyramidal
According to VSEPR theory, the terminal atom locations (“X” in Figure 3) are equivalent within the linear, trigonal planar, and tetrahedral electron-pair geometries. It does not matter which X is replaced with a lone pair because the molecules can be rotated to convert positions.
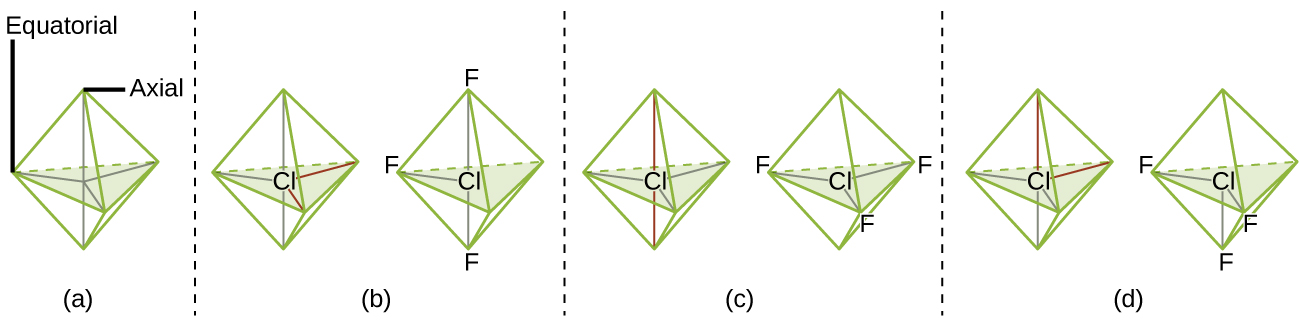
For trigonal bipyramidal electron-pair geometries, however, there are two distinct X positions, as shown in Figure 3: an axial position (if we hold a model of a trigonal bipyramid by the two axial positions, we have an axis around which we can rotate the model) and an equatorial position (three positions form an equator around the middle of the molecule). As shown in Figure 3, the axial position is surrounded by bond angles of 90°, whereas the equatorial position has more space available because of the 120° bond angles. In a trigonal bipyramidal electron-pair geometry, lone pairs or larger atoms or groups tend to occupy equatorial positions rather than axial positions because the equatorial positions are more spacious and minimize electron repulsion.
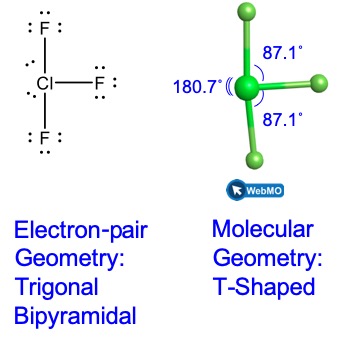
Theoretically, we can come up with three possible arrangements for the three bonds and two lone pairs for the ClF3 molecule (Figure 4). The stable structure is the one that puts the lone pairs in equatorial locations, giving a T-shaped molecular geometry.
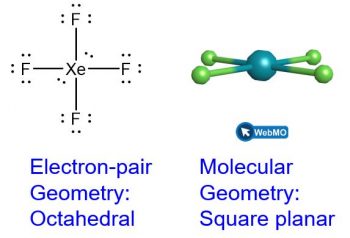
 When a central atom has two lone electron pairs and four bonding regions, we have an octahedral electron-pair geometry. The two lone pairs are on opposite sides of the octahedron (180° apart), giving a square planar molecular geometry that minimizes lone pair-lone pair repulsions (Figure 3).
When a central atom has two lone electron pairs and four bonding regions, we have an octahedral electron-pair geometry. The two lone pairs are on opposite sides of the octahedron (180° apart), giving a square planar molecular geometry that minimizes lone pair-lone pair repulsions (Figure 3).
Predicting Electron-pair Geometry and Molecular Geometry
The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular geometries:
- Write the Lewis structure of the molecule or polyatomic ion.
- Count the number of regions of electron density (lone pairs and bonds) around the central atom. A single, double, or triple bond counts as one region of electron density.
- Identify the electron-pair geometry based on the number of regions of electron density: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral (Figure 3).
- Use the number of lone pairs to determine the molecular geometry (Figure 3). If more than one arrangement of lone pairs and chemical bonds is possible, choose the one that will minimize repulsions, remembering that lone pairs occupy more space than multiple bonds, which occupy more space than single bonds. In trigonal bipyramidal arrangements, repulsion is minimized when every lone pair is in an equatorial position. In an octahedral arrangement with two lone pairs, repulsion is minimized when the lone pairs are on opposite sides of the central atom.
The following examples illustrate the use of VSEPR theory to predict the molecular geometry of molecules or ions that have no lone pairs of electrons. In this case, the molecular geometry is identical to the electron pair geometry.
Example 2: Predicting Electron-pair Geometry and Molecular Geometry: CO2 and BH3
Predict the electron-pair geometry and molecular geometry for each of the following:
- carbon dioxide, CO2
- borane, BH3
Solution
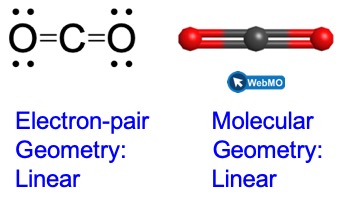 (a) We write the Lewis structure of CO2 as:
(a) We write the Lewis structure of CO2 as:
This shows us two regions of high electron density around the carbon atom—each double bond counts as one region, and there are no lone pairs on the carbon atom. Using VSEPR theory, we predict that the two regions of electron density arrange themselves on opposite sides of the central atom with a bond angle of 180°. The electron-pair geometry and molecular geometry are identical, and CO2 molecules are linear.
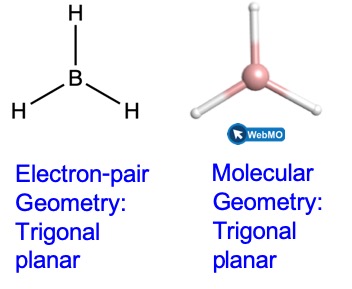 (b) We write the Lewis structure of BH3 as:
(b) We write the Lewis structure of BH3 as:
Thus we see that BH3 contains three bonds, and there are no lone pairs of electrons on boron. The arrangement of three regions of high electron density gives a trigonal planar electron-pair geometry. The B–H bonds lie in a plane with 120° angles between them. BH3 also has a trigonal planar molecular geometry.
Check Your Learning
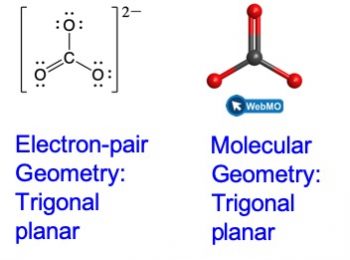
Carbonate, CO32−, is a common polyatomic ion found in various materials from eggshells to antacids. What are the electron-pair geometry and molecular geometry of this polyatomic ion?
Example 3: Predicting Electron-pair Geometry and Molecular Geometry: Ammonium
Two of the top 50 chemicals produced in the United States, ammonium nitrate and ammonium sulfate, both used as fertilizers, contain the ammonium ion. Predict the electron-pair geometry and molecular geometry of the NH4+ cation.
Solution
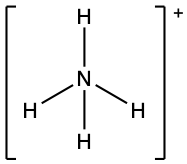 We write the Lewis structure of NH4+ as:
We write the Lewis structure of NH4+ as:
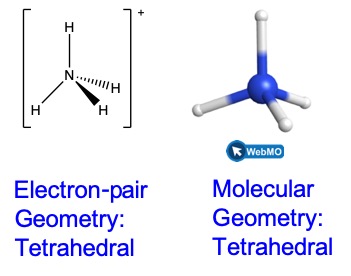 We can see that NH4+ contains four bonds from the nitrogen atom to hydrogen atoms and no lone pairs. We expect the four regions of high electron density to arrange themselves so that they point to the corners of a tetrahedron with the central nitrogen atom in the middle (Figure 3). Therefore, the electron pair geometry of NH4+ is tetrahedral, and the molecular geometry is also tetrahedral.
We can see that NH4+ contains four bonds from the nitrogen atom to hydrogen atoms and no lone pairs. We expect the four regions of high electron density to arrange themselves so that they point to the corners of a tetrahedron with the central nitrogen atom in the middle (Figure 3). Therefore, the electron pair geometry of NH4+ is tetrahedral, and the molecular geometry is also tetrahedral.
Check Your Learning
Identify a molecule with trigonal bipyramidal molecular geometry.
Answer
Any molecule with five electron pairs around the central atoms including no lone pairs will be trigonal bipyramidal. PF5 is a common example.
The next several examples illustrate the effect of lone pairs of electrons on molecular geometry.
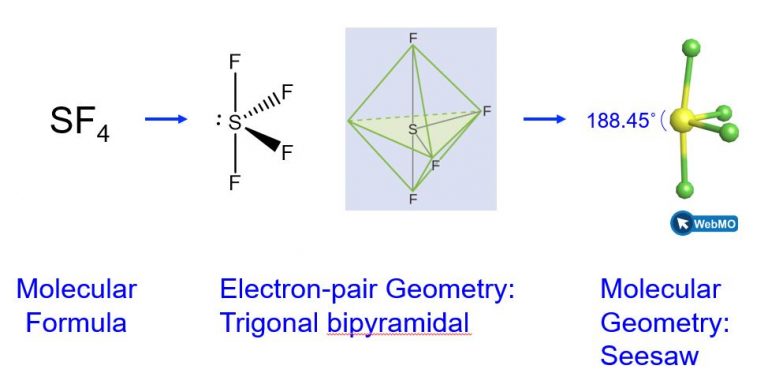
Example 4: Predicting Electron-pair Geometry and Molecular Geometry: SF4
Sulfur tetrafluoride, SF4, is extremely valuable for the preparation of fluorine-containing compounds used as herbicides (i.e., SF4 is used as a fluorinating agent). Predict the electron-pair geometry and molecular geometry of a SF4 molecule.
Solution
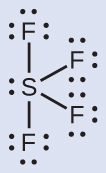 The Lewis structure of SF4 indicates five regions of electron density around the sulfur atom: one lone pair and four bonding pairs:
The Lewis structure of SF4 indicates five regions of electron density around the sulfur atom: one lone pair and four bonding pairs:
We expect these five regions to adopt a trigonal bipyramidal electron-pair geometry. In the typical fashion, the lone pair occupies one of the equatorial positions, which leads to a trigonal bipyramidal electron-pair geometry and the molecular geometry is that of a seesaw (Figure 3).
Check Your Learning
Predict the electron pair geometry and molecular geometry for molecules of XeF2.
Answer
The electron-pair geometry is trigonal bipyramidal. The molecular geometry is linear.
Example 5: Predicting Electron-pair Geometry and Molecular Geometry: XeF4
Of all the noble gases, xenon is the most reactive, frequently reacting with elements such as oxygen and fluorine. Predict the electron-pair geometry and molecular geometry of the XeF4 molecule.
Solution
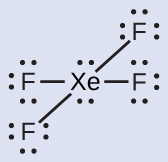 The Lewis structure of XeF4 indicates six regions of high electron density around the xenon atom: two lone pairs and four bonds:
The Lewis structure of XeF4 indicates six regions of high electron density around the xenon atom: two lone pairs and four bonds:
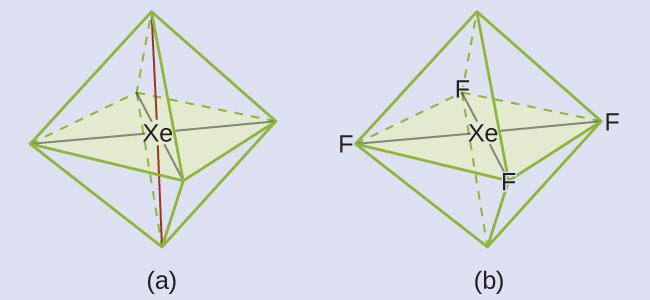 These six regions adopt an octahedral arrangement (Figure 3), which is the electron-pair geometry. To minimize repulsions, the lone pairs should be on opposite sides of the central atom. The five atoms are all in the same plane and have a square planar molecular geometry.
These six regions adopt an octahedral arrangement (Figure 3), which is the electron-pair geometry. To minimize repulsions, the lone pairs should be on opposite sides of the central atom. The five atoms are all in the same plane and have a square planar molecular geometry.
Check Your Learning
In a certain molecule, the central atom has three lone pairs and two bonds. What will the electron pair geometry and molecular geometry be?
Answer
electron pair geometry: trigonal bipyramidal; molecular geometry: linear
Molecular Geometry for Multi-center Molecules
When a molecule or polyatomic ion has only one central atom, the molecular geometry completely describes the shape of the molecule. Larger molecules do not have a single central atom, but are connected by a chain of interior atoms that each possess a “local” geometry. The way these local structures are oriented with respect to each other also influences the molecular shape, but such considerations are largely beyond the scope of this introductory discussion. For our purposes, we will only focus on determining the local structures.
Example 6: Predicting Structure in Multicenter Molecules
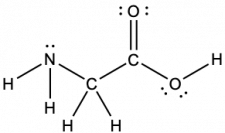 The Lewis structure for the simplest amino acid, glycine, H2NCH2CO2H, is shown here. Predict the local geometry for the nitrogen atom, the two carbon atoms, and the oxygen atom with a hydrogen atom attached:
The Lewis structure for the simplest amino acid, glycine, H2NCH2CO2H, is shown here. Predict the local geometry for the nitrogen atom, the two carbon atoms, and the oxygen atom with a hydrogen atom attached:
Solution
Consider each central atom independently. The electron-pair geometries:
- nitrogen––four regions of electron density, tetrahedral
- carbon (CH2)––four regions of electron density, tetrahedral
- carbon (CO2)—three regions of electron density, trigonal planar
- oxygen (OH)—four regions of electron density, tetrahedral
The local structures:
- nitrogen––three bonds, one lone pair, trigonal pyramidal
- carbon (CH2)—four bonds, no lone pairs, tetrahedral
- carbon (CO2)—three bonds (double bond counts as one region of electron density), no lone pairs, trigonal planar
- oxygen (OH)—two bonds, two lone pairs, bent (109°)
Check Your Learning
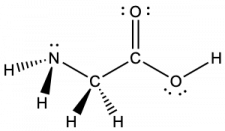
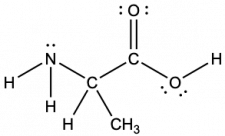 Another amino acid is alanine, which has the Lewis structure shown here. Predict the electron-pair geometry and local structure of the nitrogen atom, the three carbon atoms, and the oxygen atom with hydrogen attached:
Another amino acid is alanine, which has the Lewis structure shown here. Predict the electron-pair geometry and local structure of the nitrogen atom, the three carbon atoms, and the oxygen atom with hydrogen attached:
Answer:
electron-pair geometries:
- nitrogen—tetrahedral
- carbon (CH)—tetrahedral
- carbon (CH3)—tetrahedral
- carbon (CO2)—trigonal planar
- oxygen (OH)—tetrahedral
local structures:
- nitrogen—trigonal pyramidal
- carbon (CH)—tetrahedral
- carbon (CH3)—tetrahedral
- carbon (CO2)—trigonal planar
- oxygen (OH)—bent