D10.1 Temperature and Solubility
Can you also predict the effect of temperature on solubility? If you raise the temperature, does solubility of a solute increase or decrease? It would be reasonable to assume that increasing temperature increases solubility. But remember that both ΔH and ΔS have a role, and an increase in temperature increases the effect of changes in entropy. Dissolving solute into solvent is likely to increase entropy (if ΔS is positive), but this is not always the case. Consider what happens when you heat up water on the stove. Bubbles of gas are released from the liquid long before the water reaches its boiling point. At low temperatures, these bubbles contain air (primarily N2, O2) that was dissolved in the water. (At the boiling point, the bubbles contain only water molecules because all the air has been expelled long before this temperature is reached.) Why? Because the solubility of most gases in water decreases as temperature rises. We can trace the reason for this back to the entropy of solution. Most gases have very small intermolecular attractions – this is the reason why they are gases after all. Gas molecules do not stick together and form solids and liquids. Therefore, they do not have very high solubility in water. As an example, the solubility of O2 in water is 8.3 mg/L (at 25 ºC and 1 atmosphere).
Most gases have a slightly favorable (negative) enthalpy of solution and a slightly unfavorable (negative) entropy of solution. The effect on enthalpy can be traced to the dipole–induced dipole attractions formed when the gas dissolves in the solution.
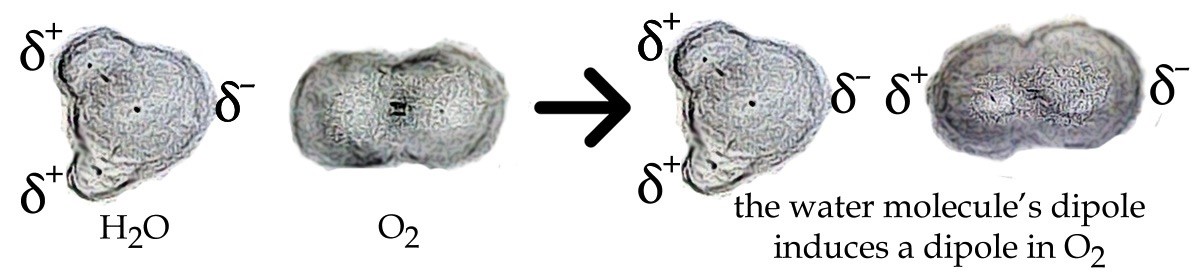
The decrease in entropy results from the fact that the gas molecules are no longer free to roam around – their positional entropy is more constrained within the liquid phase than it is in the gas phase. When the temperature is increased the gas molecules have more kinetic energy and therefore more of them can escape from the solution, increasing their entropy as they go back to the gas phase. Thus, the solubility of O2 and other gases decreases as temperature increases. This can produce environmental problems, because less oxygen is available for organisms that live in the water. A common source of thermal pollution occurs when power plants and manufacturing facilities expel warm water into the environment.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

