D4.4 Conformations
At room temperature, molecules are in constant motion both with respect to other molecules and as a result of internal motions such as rotation of one part of a molecule relative to another part of the same molecule. Below is an animation of a butane molecule at room temperature. Notice that there is rotation around the central C–C single bond (which is kept stationary in the animation to show the rotation better) and also around the other C–C single bonds. Such rotations in a molecule lead to different conformations or conformers, structures that differ only because of rotations around single bonds.
As you can see in the above animation, no bond breaking is needed to go from one conformer to another. Because the energy required for rotation around single bonds is small, such rotations occur readily at room temperature for most molecules. Hence, at room temperature, one conformer cannot be isolated from another and chemists consider that conformers represent the same chemical compound, with the same name and the same physical properties.
Activity: Depicting Conformers
In the animation of a nonane molecule below, you can see that different conformers of the same molecule can adopt, at a first glance, dramatically different molecular shapes. If you change your viewpoint by moving the molecule with your mouse, you can see that the molecule also looks quite different from different perspectives.
Given two or more Lewis structures, it is important that you can recognize whether they are conformers or isomers. Lewis structures or line structures that look quite different may very well be different conformers or the same conformer drawn from different perspectives; in either case, the structures would represent the same substance.
A good way to tell whether two structures represent the same substance is to work out the correct name of each structure. If the names are the same, the structures represent conformers, not different substances. You will not be explicitly tested on naming compounds (nomenclature) in this course, but we strongly recommend that you study the section about alkanes in the appendix Organic Nomenclature. IUPAC names are a systematic way of naming chemical compounds that can also help you when thinking about molecular structure. Going forward, if you encounter a name for a chemical compound that’s unfamiliar to you, the Organic Nomenclature appendix can help.
Activity: Identifying Conformers
Consider these four line structures.
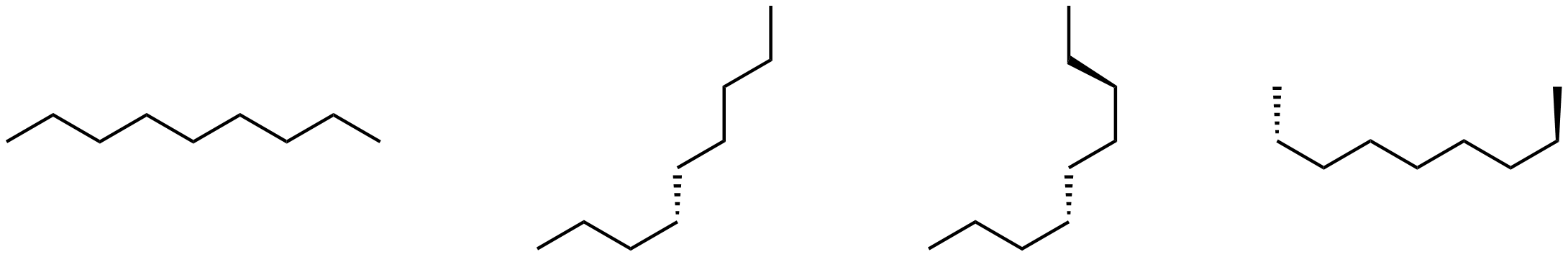
Which, if any, represents a different compound? Name each structure to see whether it differs from the others.
Write in your notebook, then left-click here for an explanation.
To name a structure, determine the longest chain of carbon atoms and name the compound based on the number of carbon atoms in the longest chain. Then identify any branches (side chains), name them, and indicate their positions by numbering the C atoms in the longest chain to give the lowest numbers.
Each of the structures consists of nine carbon atoms in a chain. There are no branches. Thus each structure is named nonane and all structures represent the same compound. The structures are different conformations of nonane.
While the different conformers represent the same chemical compound, there are instances where specific conformations matter. For example, for some compounds, different conformations can have different reactivity in a specific reaction. And on a larger scale, huge protein molecules adopt only a few of many possible conformations; the shapes of protein molecules are essential to their biological functions.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

