D10.4 Rate Constant
The rate of a chemical reaction is usually defined as the change in concentration of a reactant or a product per unit time. Different substances react at different rates. Moreover, for most reactions, the rate of the reaction is dependent on one or more reactant concentrations:
Here, k is the rate constant, a proportionality constant independent of reactant concentrations that is specific to a particular reaction at a particular temperature. (We will revisit this equation later and discuss the implication of the exponents.) Because k is independent of reactant concentrations, it is a useful value when comparing rates of different reactions.
Just like the equilibrium constant, the rate “constant” is only constant at a given temperature. (Note that the equilibrium constant is a upper-case “K”, while the rate constant is a lower-case “k”.) In our molecular-level exploration of chemical reactions, we have already discussed qualitatively several aspects of a reaction that would influence its rate. To summarize, there are three main factors that determines the value of k, and the first two depend on temperature:
- To react, molecules must come close enough to each other to exchange energy and perhaps to break and form bonds; that is, molecules must collide. The rate constant is proportional to the rate of collisions when all concentrations are 1 M:
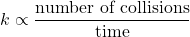
- For a reaction to occur, there must be sufficient energy in the reactant molecule or molecules to allow electrons to rearrange to break and/or form bonds. If all else are equal, a smaller energy requirement (smaller activation energy) leads to a larger rate constant.
- The reacting molecules must collide in an orientation that allows the reaction to proceed; that is, even if the molecules have enough energy, a collision is more likely to result in reaction when the molecules are oriented in certain ways with respect to each other. This is the steric factor .
Next, we will consider the relationship between rate constant and temperature quantitatively.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

