D1.4 Alkenes
Unsaturated hydrocarbons that contain one or more double bonds are called alkenes. The general molecular formula for alkenes with one double bond is CnH2n. The formula has two hydrogen atoms fewer than the corresponding alkane with same number of carbon atoms, and hence there is one degree of unsaturation. It is possible to have a ring of carbon atoms that contains a double bond. Cyclic alkenes have one degree of unsaturation from each cyclic structure and one from each C=C double bond.
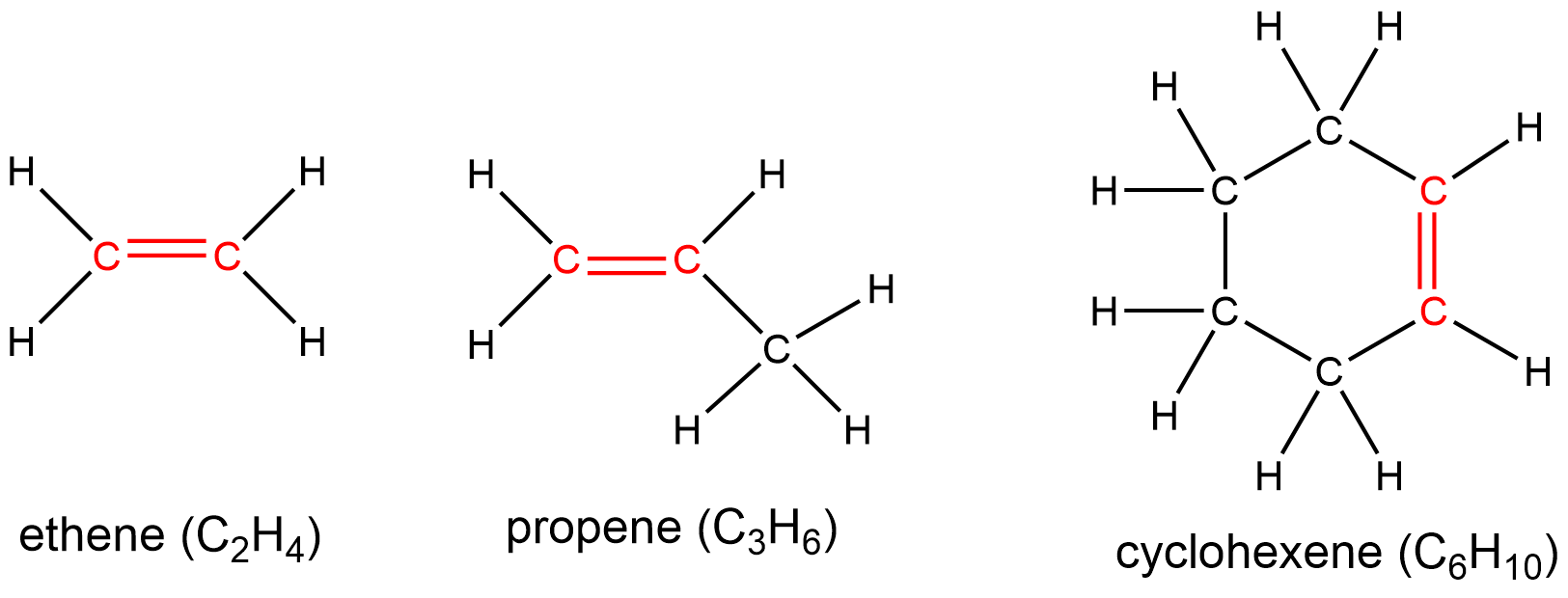
The presence of these double bond(s) is signified by the suffix -ene in the name. Ethene, C2H4, commonly called ethylene, is the simplest alkene. Some other examples include:
A C=C double bond consists of a σ bond and a π bond. The presence of the π bond makes alkenes much more reactive than alkanes (which only have σ bonds) because the π bond is usually weaker and more easily disrupted than a σ bond. The double bond is therefore a functional group, a specific structure that has similar chemical behavior in every molecule where it occurs. For example, all alkenes can undergo a characteristic reaction, called an addition reaction, in which the double bond is partially broken and two new single bonds form. Hydrogen and halogen molecules can undergo addition reactions with alkenes, for example:

In this reaction, the π bond in the C=C double bond (red) and the Cl-Cl σ bond (red) are broken, and two C-Cl σ bonds (green) are formed. The σ bond in the C=C double bond remains intact throughout the reaction. The reaction can occur relatively easily because the carbon-carbon π bond is not as strong as the carbon-carbon σ bond, that is, the C=C double bond (average bond energy = 598 kJ/mol) is not twice as strong as the C-C single bond (average bond energy = 346 kJ/mol).
Activity: Analyzing an Addition Reaction
Think about the reaction of chlorine with ethane, CH3CH3. Can this be an addition reaction? Explain why or why not. What bonds need to be broken and formed if chlorine reacts with ethane and how does the reaction differ from the reaction of chlorine with ethene shown above? Do you expect the reaction of chlorine with ethane to be faster or slower than the reaction with ethene? Why?
Write in your notebook, then left-click here for an explanation.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

