D4.3 Aromatic Molecules
Benzene, C6H6, is representative of a large number of aromatic compounds. These compounds contain ring structures and exhibit bonding that must be described using resonance structures. The resonance structures for benzene are:
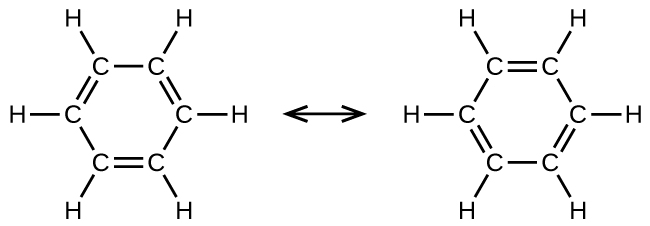
All six carbon-carbon bonds are equivalent and exhibit bond lengths that are intermediate between those of a C–C single bond and a C=C double bond.
The chemical reactivity of aromatic compounds differs from the reactivity of alkenes. For example, aromatic compounds do not undergo addition reactions. Instead, with the aid of a catalyst, they can undergo substitution reactions where one of the hydrogen atoms is replaced by a substituent: another atom or group of atoms. A substitution reaction leaves the delocalized double bonds intact.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

