D7.2 Chemical Equilibrium
To begin our journey toward understanding chemical reactions, we consider chemical equilibrium. When a chemical reaction takes place in the gas phase in a closed container or in a liquid solution, concentrations of reactants and products change as the reaction proceeds. The reaction eventually reaches a dynamic chemical equilibrium.
Consider this example. Place 0.10 mol nitrogen dioxide (NO2, a red-brown gas) in a 1.00-L glass flask at room temperature. The NO2 reacts rapidly to form dintrogen tetraoxide (N2O4, a colorless gas) according to this equation:
2 NO2(g) ⟶ N2O4(g)
red-brown ⟶ colorless
As the reaction occurs, the concentration of NO2 decreases and so the intensity of red-brown color also decreases. But some color remains, implying that not all of the NO2 has reacted away. The reaction apparently stops before all reactant has been converted to product; it has reached chemical equilibrium.
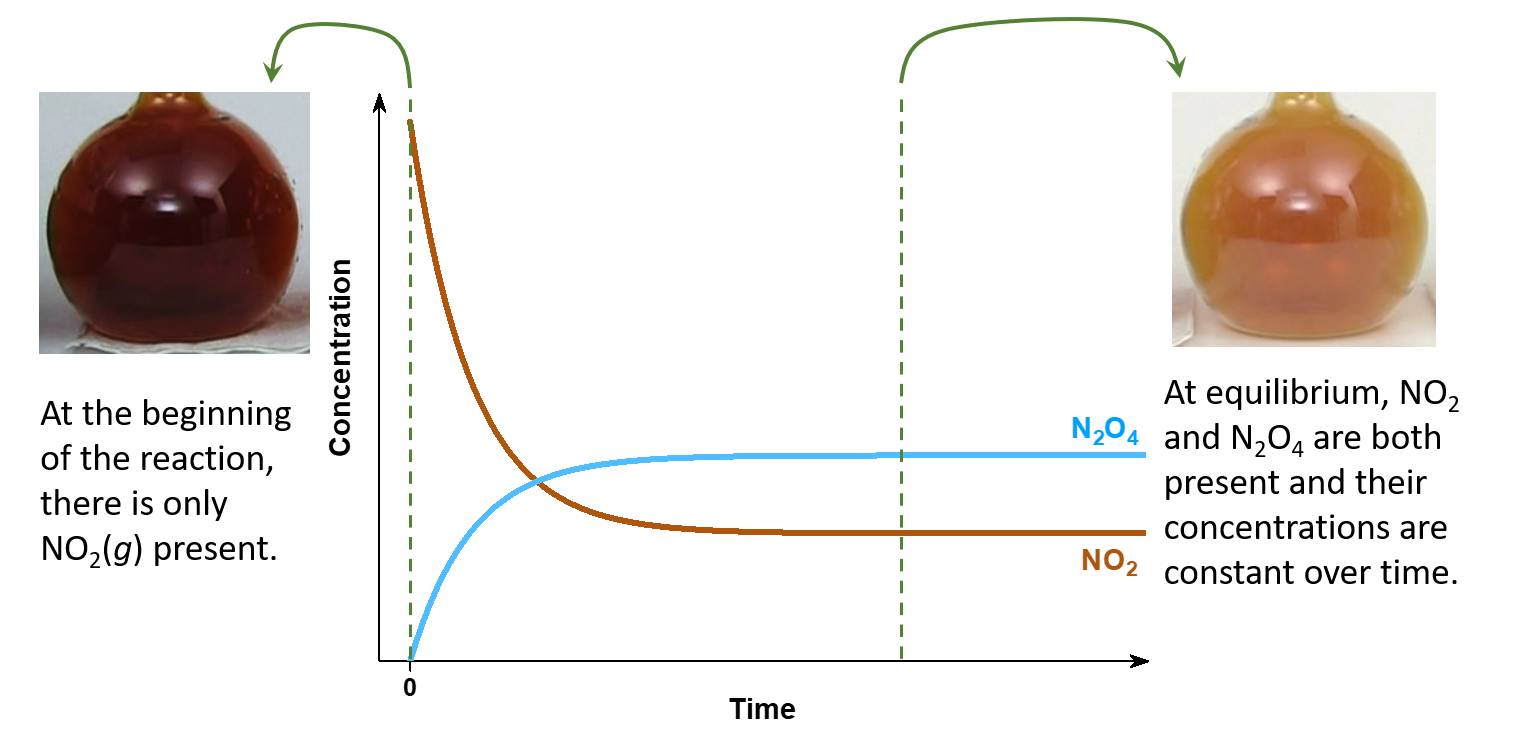
In a separate experiment, place 0.05 mol N2O4 in a 1.00-L flask at room temperature. A pale red-brown color appears, indicating that the N2O4 has reacted to form NO2 according to this equation:
N2O4(g) ⟶ 2 NO2(g)
colorless ⟶ red-brown
Only a little color appears when equilibrium is reached, indicating that only a little NO2 forms. Measurements with a spectrometer show that the intensity of color at equilibrium is identical in both experiments.
Activity: Approaching Chemical Equilibrium
Our discussion so far indicates that NO2 can react to form N2O4 and that N2O4 can react to form NO2. The reaction can be written this way:
2 NO2(g) ⇌ N2O4(g)
red-brown ⇌ colorless
where the double arrow, ⇌, indicates that both reactions can occur at the same time. That is, the reaction is reversible, whether or not it has reached equilibrium. Based on the way the third equation has been written, the first two equations above are called the forward reaction and the reverse reaction, respectively.
If you were able to view this reaction at the atomic scale, you would see that even after equilibrium has been achieved both the forward reaction and the reverse reaction continue to occur. Concentrations stop changing when the rate of the forward reaction equals the rate of the reverse reaction—that is, when the reaction of N2O4 to form NO2 is just as fast as the reaction of NO2 to form N2O4—but that does not mean that the reactions stop. The fact that both reactions continue to occur is the reason this is called a dynamic chemical equilibrium.
Exercise: Analyzing a Chemical Equilibrium
For a chemical equilibrium,
- Forward and reverse reactions occur at the same rate, and the concentrations of products and reactants remain constant over time.
- The same equilibrium concentrations can be achieved by starting with reactant(s) only, product(s) only, or a mixture of reactant(s) and product(s), provided that the same total number of atoms of each kind is present.
These two features provide a way to test whether a system is at chemical equilibrium.
Activity: Is a System at Dynamic Equilibrium?
Suppose that 1.00 mol H2(g) and 1.00 mol I2(g) are introduced into a 10.0-L flask at 500 K. After 10 min the flask contains 1.72 mol HI(g), 0.14 mol H2(g) and 0.14 mol I2(g). A fellow student says that the reaction has reached equilibrium. After waiting another 10 min the amounts of reactants and products remain the same.
Write a balanced chemical equation for the equilibrium reaction.
Describe an experiment you could do to verify that the reaction is at equilibrium. Give sufficient details, such as amounts of reactants or products, such that the experiment would be feasible to perform as described.
Write in your notebook, then left-click here for an explanation.
The equilibrium equation is
H2(g) + I2(g) ⇌ 2 HI(g)
There are several possible experiments. One is to start with the product, HI, in sufficient amount so that the same number of H atoms and I atoms are present, and see whether the same amounts of HI, H2, and I2 are formed. The appropriate amount of HI is 2.00 mol.
A second possible experiment is to start with a mixture of H2, I2 , and HI that contains the same number of H atoms and I atoms as in the initial experiment. For example, the experiment could start with 0.50 mol H2, 0.50 mol I2, and 1.00 mol HI. If this mixture reacts to form 1.72 mol HI and 0.14 mol each of H2 and I2, then the original system was at equilibrium.
When 0.00001 mol D2, hydrogen consisting of two atoms of deuterium, the isotope with mass number 2, is introduced into the equilibrium system described above, mass spectrometric analysis of the equilibrium mixture about 10 min later reveals that nearly all the deurerium is found in DI and HD molecules. Almost none remains as D2.
Write an explanation for how this information supports the idea of dynamic chemical equilibrium.
Write in your notebook, then left-click here for an explanation.
Assume that deuterium behaves chemically the same way as ordinary hydrogen. (This assumption has been verified by past science.)
The amount of D2 added to the system is negligible compared to the equilibrium amounts of the reactants and products so the equilibrium should not be affected by this small addition.
The fact that very little D2 remains after 10 min indicates that the forward reaction,
D2 + I2 → 2 DI
is occurring. It is reasonable to assume that the forward reaction involving ordinary hydrogen is also continuing to occur.
Formation of HD indicates that the reverse reaction must also be occurring. The amount of D2 added to the equilibrium system is extremely small, so once DI forms a DI molecule is much more likely to encounter a HI molecule than to react with another DI molecule. The reverse reaction is then
DI + HI → HD + I2
Thus a dynamic equilibrium accounts for the experimental facts.
An equilibrium can be established for a physical change as well as for a chemical reaction. For example:
The figure below shows a sample of liquid Br2 at equilibrium with Br2 vapor in a closed container. When we pour liquid Br2 into an empty bottle in which there is no bromine vapor, some liquid evaporates: the amount of liquid decreases and the amount of vapor increases. If we seal the container so no vapor escapes, the amount of liquid and vapor will eventually stop changing; at that point an equilibrium between the liquid and the vapor has been established. If the container were not sealed, the bromine vapor would escape and no equilibrium would be reached.

Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

