D11.6 Competing Reactions
Kinetic metastability can be used to our advantage in the case of competing reactions, where more than a single chemical transformation can take place at the same time. Often it is possible to select conditions under which the reaction that is less thermodynamically favored is the main reaction. For example, consider the reaction below.
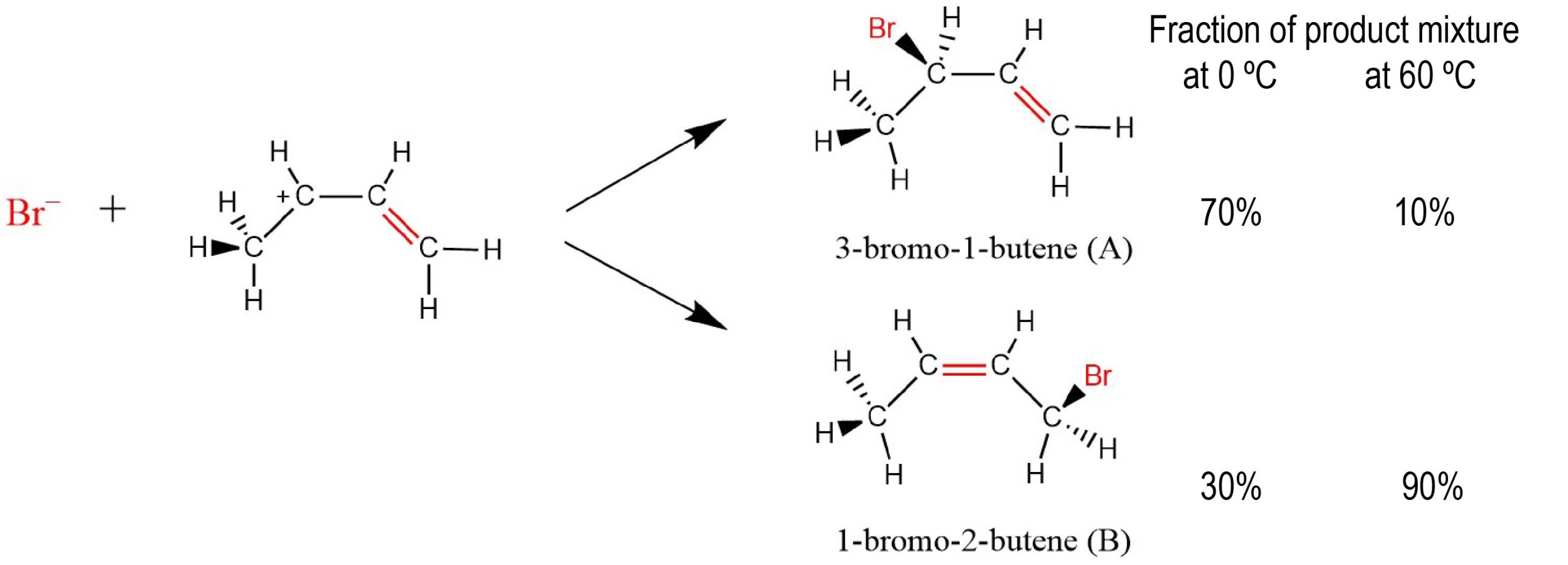
Notice that at 0 °C most of the product is 3-bromo-1-butene (compound A), but at 60 °C most of the product is 1-bromo-2-butene (compound B). Why are two different products formed? Why should temperature make such a difference in how much of each is formed?
Activity: Why Are Two Different Products Formed?
The two products are formed because there are two positive sites in the resonance hybrid for this cation. Now let us explore why temperature makes a difference regarding which product predominates. We can understand this better using a reaction energy diagram like the one in Figure: Reaction energy diagram for competing reactions.
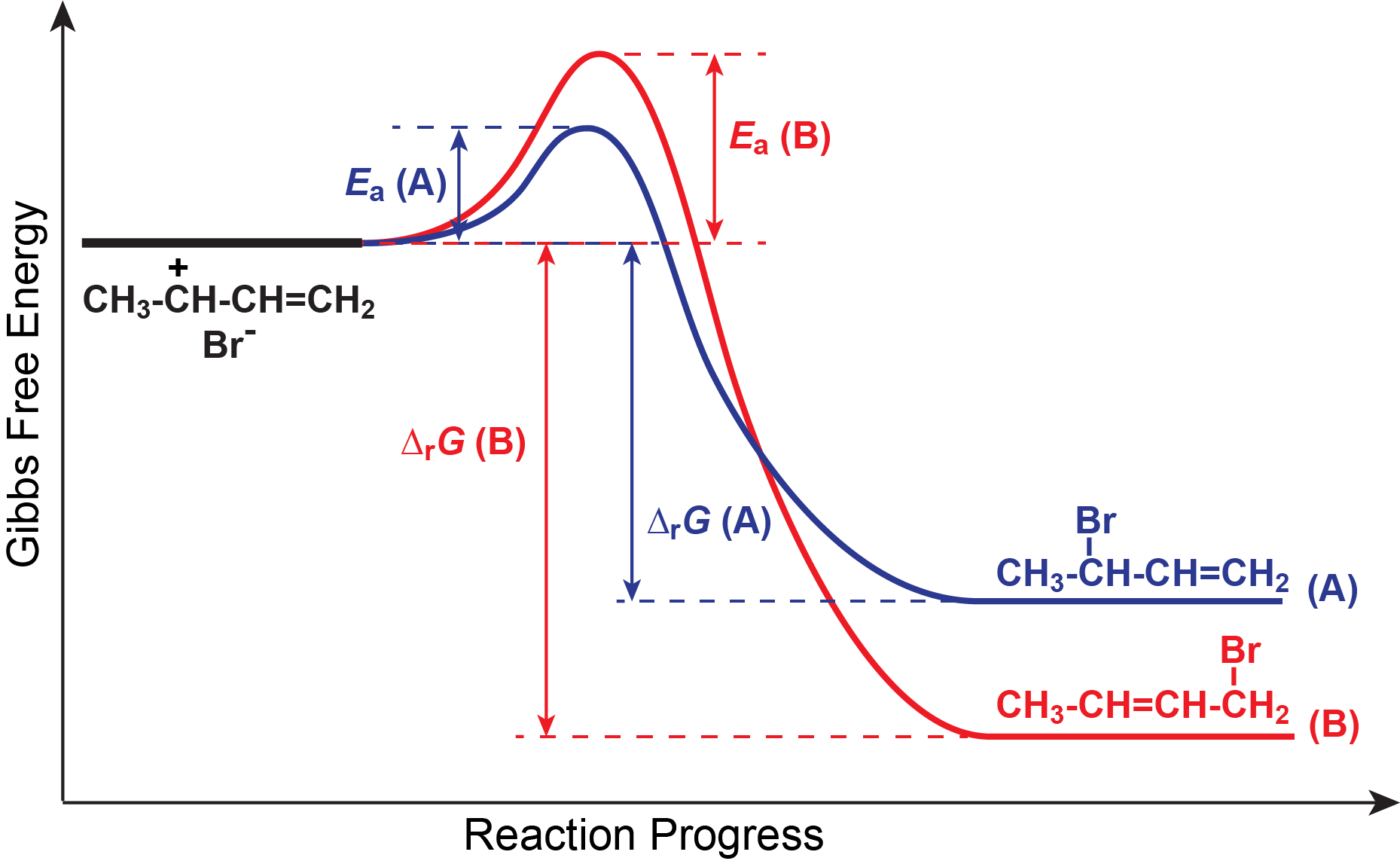
Note that the activation energy for reaction A is lower than for reaction B, and the negative Gibbs free energy change for reaction A is smaller than for reaction B. The reaction energy diagram can be used to infer several features of these two reactions. For example, compound B is thermodynamically stable relative to compound A; however, there is a large energy barrier (the activation energy of the reverse reaction of compound A) that can prevent compound A from converting to compound B. Thus, there is a good possibility that under certain conditions compound A is kinetically metastable relative to compound B.
Comparing activation energies, we find that the path to compound A is more accessible at low temperatures because of the lower activation energy, but that the increase in rate constant as temperature increases will be faster for the path to compound B because it has a larger activation energy. These ideas enable us to explain the fact that a greater percentage of the product at 0 °C is compound A, but compound B predominates at 60 °C.
At 0 °C only a very few molecules have energy equal to the activation energy for compound B. More molecules have enough energy to overcome the smaller activation barrier to reach compound A. However, once compound A forms the activation barrier for the reverse reaction is so large that essentially no molecules have enough energy to react back to reactants. We refer to the reaction that forms compound A as irreversible because the reverse reaction essentially does not occur.
As temperature rises, the fraction of molecules with high energies increases rapidly. The first effect is to enable the reaction that produces B, even though it has a higher activation energy than the reaction that produces A. And the rate constant for the reaction that produces B increases more rapidly with temperature than the rate constant for A. Thus, B is being produced faster and faster and the rate constant for producing B is going up faster than for A. This results in an increasing fraction of B being produced.
At high enough temperature there are enough molecules with sufficient energy to overcome the activation barriers to the the reverse reactions. Once this happens the forward reactions are no longer irreversible and the entire system can reach equilibrium. There is a large negative ΔrG for each reaction (and therefore a large K for each reaction) so nearly all of the reactant molecules are changed to product molecules. Because compound B is thermodynamically stable relative to compound A, at equilibrium the concentration of compound B is larger than the concentration of compound A and the product mixture contains 90% B and only 10% A.
Thus, under conditions where the forward reactions are irreversible, the ratio of products is controlled by the rates of the reactions and because the rate of the reaction that produces A is faster, the product mixture contains more A. We refer to this situation, where the product mixture is determined by reaction rates, as kinetic control of the reaction. When all reactions occur fast enough so that equilibrium can be achieved, the reaction system is said to be under thermodynamic control.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

