D6.5 Conjugated-Diene Polymers
Conjugated dienes (alkenes with a double bond-single bond-double bond configuration) can be polymerized to form important substances, such as rubber. This occurs in nature as well as in the laboratory. The simplest conjugated diene is 1,3-butadiene; the figure below shows the 1,4-polymerization of this monomer. In the resulting polymer, a new σ bond (highlighted in red) is formed between carbon 1 of one monomer and carbon 4 of another monomer, and within each monomer, a π bond is moved to between carbon 2 and carbon 3.
During the polymerization reaction: one electron from the C1=C2 π bond in monomer B pairs with an electron from an adjacent monomer A to form a new σ bond involving C1; similarly, one electron from the C3=C4 π bond in monomer B pairs with an electron from another adjacent monomer C to form a new σ bond involving C4; the other electron from each π bond moves to the center of the molecule, and forms a new π bond between C2 and C3.
Activity: 1,4-Addition Polymerization
Natural rubber is formed from the monomer isoprene (2-methyl-1,3-butadiene):
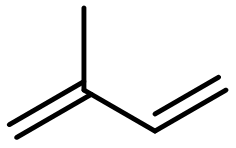
Natural rubber is obtained as a milky white fluid known as latex. Most of the double bonds in the polymer chain have the cis configuration.
In your notebook, draw the structure of natural rubber with at least two repeating units. Indicate which are the new bonds that form as the polymer chain lengthens.
Draw in your notebook, then left-click here for an explanation.
Polymerization reaction steps occur at the ends of the 2-methyl-1,3-butadiene molecule (at the 1-carbon and the 4-carbon atoms); the new bonds are colored red in the structure below.
Double bonds are formed between the 2- and 3-carbon atoms, using p orbitals that were originally involved in the two double bonds.
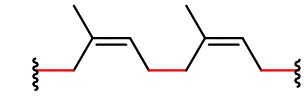
Chemical reactions involving double bonds on adjacent polymer strands can lead to cross-linking, which enhances elasticity of the polymer. In 1839, Charles Goodyear discovered that when natural rubber was heated to 140–160 °C in the presence of sulfur, the rubber became tougher, more resistant to heat and cold, and more elastic. This process was later called vulcanization after Vulcan, the Roman god of fire and volcano. Above 140 °C, S–S bonds in sulfur molecules, S8, break, and linear chains of sulfur atoms form. These chains then react with some of the remaining double bonds in the polymer, forming cross links. The development of vulcanized rubber for automobile tires greatly aided the automobile industry.
Another important conjugated diene used in synthetic rubber is chloroprene (2-chloro-1,3-butadiene). Polymerized chloroprene was developed by DuPont and given the trade name Neoprene. Cross-linking in polychloroprene involves combination of two chlorine atoms from adjacent chains with a Zn2+ ion to form ZnCl2. The C–Cl bonds in the uncross-linked polymer lose their Cl become C–C bonds—the cross-link. Cross-linking contributes to the overall elasticity of neoprene.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

