D16.1 Definition of Acids and Bases
We are now in a position to apply knowledge developed in earlier units to explore an important and very common class of chemical reactions: acid-base reactions. First, we need to define what is an acid and what is a base. There are two related definitions.
Brønsted-Lowry
In 1923, the Danish chemist Johannes Brønsted and the English chemist Thomas Lowry proposed the Brønsted-Lowry acid-base definition. According to this definition, a chemical species that donates a proton (hydrogen ion, H+) to another chemical species is called an acid and a chemical species that accepts a proton is a base. (Recall that when a H atom loses an electron, only the proton in the nucleus remains, so a proton is a H+ ion.) An acid-base reaction is the transfer of a proton from a proton donor (acid) to a proton acceptor (base).
The chemical species that remains after an acid has donated a proton is called the conjugate base of that acid. Consider these examples of the generic reaction, acid + H2O ⇌ conjugate base + H3O+:
H2SO4(aq) + H2O(ℓ) ⇌ HSO4–(aq) + H3O+(aq)
HSO4–(aq) + H2O(ℓ) ⇌ SO42-(aq) + H3O+(aq)
NH4+(aq) + H2O(ℓ) ⇌ NH3(aq) + H3O+(aq)
Similarly, the chemical species that forms after a base accepts a proton is called the conjugate acid of that base. Consider these examples of the generic reaction, base + H2O ⇌ conjugate acid + OH–:
S2-(aq) + H2O(ℓ) ⇌ HS–(aq) + OH–(aq)
CO32-(aq) + H2O(ℓ) ⇌ HCO3–(aq) + OH–(aq)
F–(aq) + H2O(ℓ) ⇌ HF(aq) + OH–(aq)
From these examples, we can see that the conjugate base of an acid has that acid as its conjugate acid, in other words, conjugate base and conjugate acid are paired. For instance, NH3 is the conjugate base of the acid NH4+, while NH4+ is the conjugate acid of the base NH3. Similarly, F‾ is the conjugate base of HF, while HF is the conjugate acid of F–.
You may have noticed in earlier sections that we wrote H+(aq) to represent hydrogen ions in aqueous solution, while in the preceding reactions we have written H3O+ instead. A H+ ion, a proton, is much smaller than any other cation and therefore is a highly concentrated positive charge that strongly attracts water molecules and forms very strong hydrogen bonds. Thus, when a Brønsted-Lowry acid transfers a proton to water, more than a single water molecule accepts the proton. Experiments show that as many as six water molecules may be involved, which would render the formula of H+(aq) as H13O6+. However, the structure of H+(aq) changes continuously as water molecules move about in the liquid, so H13O6+ is not exact either. For Brønsted-Lowry acid-base reactions, we will use H3O+(aq) to emphasize that a proton has been transferred to water, and to represent the more complicated actual structure. In general, H+(aq) is appropriate to represent a proton surrounded by many water molecules.
Brønsted-Lowry acid-base reactions are very fast. They reach equilibrium quickly and this equilibrium behavior is central to our upcoming description of acid and base characteristics.
Lewis
At about the same time the Brønsted-Lowry acid-base definition was proposed, an even more general model of acid-base behavior was introduced by the American chemist G. N. Lewis. It centers on the electron exchanges that occur in acid-base reactions. According to the Lewis acid-base definition, an acid is a chemical species that can accept a pair of electrons; a base is a chemical species that can donate a pair of electrons.
The Brønsted-Lowry definition is included in the Lewis definition: a Brønsted-Lowry acid is also a Lewis acid, a Brønsted-Lowry base is also a Lewis base. For example, consider the reaction:
For the forward reaction, NH4+ is the Brønsted-Lowry acid because it donates a proton (highlighted in red below). NH4+ is also the Lewis acid because it accept a pair of electrons.
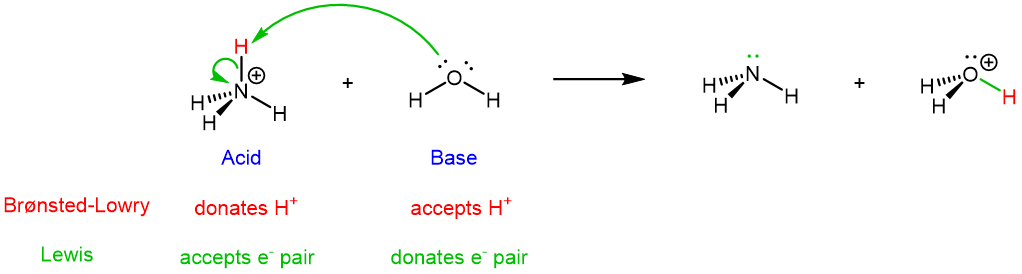
The electron movement involved in the forward reaction is shown by the green curved arrows: a lone pair on O goes to form a new O-H covalent bond, and the two electrons in the N-H bond become a lone pair on N. Note that the general flow of electrons is from H2O (base, electron donor) to NH4+ (acid, electron acceptor). The two electrons in the newly formed O-H bond, highlighted in green in H3O+, come entirely from H2O.
The idea is the same for the reverse reaction (the green curved arrows show the overall flow of electrons from base to acid):
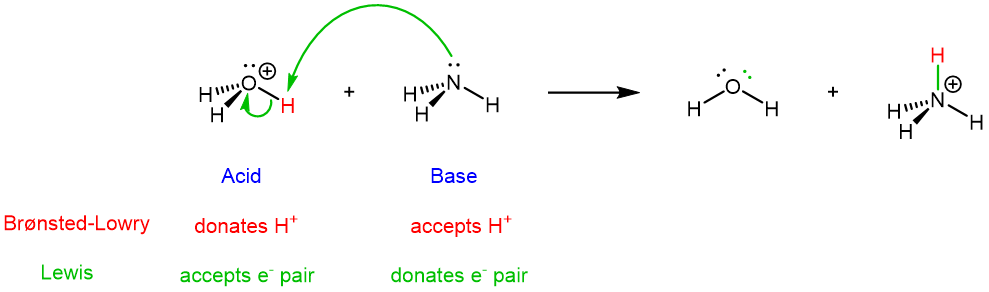
It is these movements of electrons during a chemical reaction that lead to bond breaking and bond formation, and thus the chemical transformations that occur during a reaction. Therefore, the Lewis definition, centered on electron movement, is a very powerful tool for describing chemical reactions.
The Lewis acid-base definition is broader than the Brønsted-Lowry definition: some Lewis acid-base reactions do not involve protons. For example, in the equation below BF3 acts as a Lewis acid, accepting a pair of electrons from fluoride ion, a Lewis base:
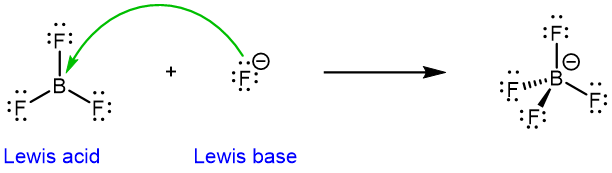
The boron in BF3 is electron deficient (it does not have a full octet). It can readily accept electrons from an electron-rich fluoride anion, making BF3 a Lewis acid. (F– is both a Lewis base and a Brønsted-Lowry base.)
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

