D23.2 Electroplating
An important application for electrolytic cells is electroplating, which forms a thin coating of metal on top of a conducting surface. Metals typically used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. As an example of electroplating, consider how silver-plated tableware is produced in the setup shown below.
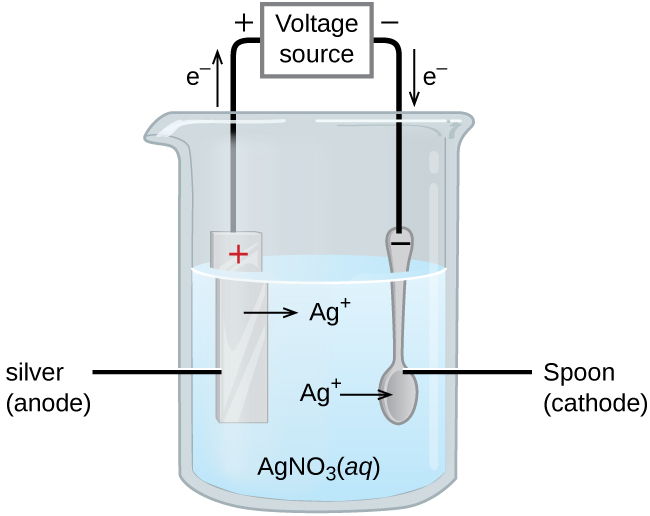
The anode consists of a silver electrode. The cathode is a spoon made from a less expensive metal. Both electrodes are immersed in a solution of silver nitrate. As the potential from the voltage source is increased, current starts to flow.
Silver metal is lost at the anode as Ag+ goes into solution:
The mass of the cathode increases as Ag+ ions from the solution are deposited onto the spoon:
The net result is the transfer of silver metal from the anode to the cathode. The quality of the electroplated object depends on the thickness of the deposited silver and the rate of deposition.
Quantitative Aspects of Electrolysis
The quantity of current that flows in an electrolytic cell is dictated by the amount (moles) of electrons transferred in a redox reaction, which is in turn related to quantities of reactants and products via reaction stoichiometry. Recall that current, I, is related to the total charge, Q, by:
Hence:
where F is the Faraday constant.
Exercise: Electroplating
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

