D6.4 Examples of Addition Polymers
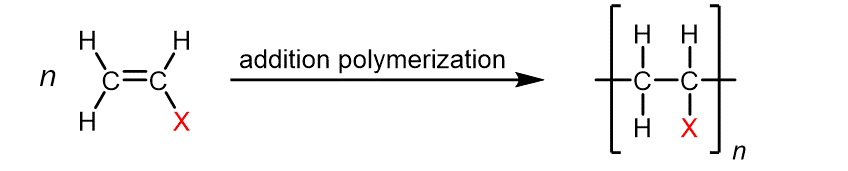
The table below lists monomers for some well-known addition polymers and examples of their uses. You can probably find at least one instance of each of them in your home. Each monomer is a variation of the ethylene (ethene) structure in which one or more H atoms has been replaced by another group (highlighted in the table). Such substitution(s) in the monomer allow the physical properties of the polymer—such as density, melting range, strength, and whether the polymer is attracted to or repelled by water—to be precisely controlled, and the polymer tailored for specialized uses. Note that in the reaction equation below, the polymer structure is specified by enclosing a single repeating unit in brackets and specifying the repeating nature by a subscript “n“.
| Monomer | Common Name | Polymer | Physical Property | Some Typical Uses |
|---|---|---|---|---|
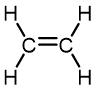 |
Ethylene | Polyethylene | Varied depending on branching/density | Packaging films, toys, bottles, coatings, water pipes |
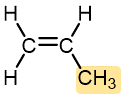 |
Propylene | Polypropylene (Herculon) | Usually tough and flexible, similar to HDPE | Hinges, molding, rope, outdoor carpeting |
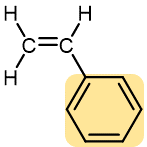 |
Styrene | Polystyrene (Styrofoam, Styron) | Hard and rather brittle | Transparent containers (such as Petri dish), plastic glasses, styrofoam |
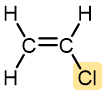 |
Vinyl chloride | Polyvinyl chloride (PVC) | Hard, moderately tough, can be rigid or flexible | Pipe and tubing, raincoats, phonograph records, floor tiles |
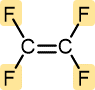 |
Tetrafluoroethylene | Polytetrafluoroethylene (Teflon) | Hard and tough | Nonstick pan coatings, bearings, gaskets |
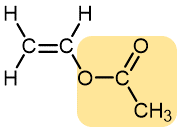 |
Vinyl acetate | Polyvinyl acetate | Highly branched, not solid at room temperature | Elmer’s glue, wood glue |
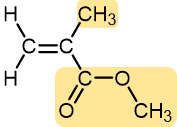 |
Methyl methacrylate | Polymethyl methacrylate (Plexiglass, Lucite) | Hard and brittle, but can be modified to be more shatter-resistant | Stiff plastic sheets, blocks, tubing, and other shapes |
The notation where a repeating unit in a polymer is shown in brackets emphasizes that it is important to be able to recognize a repeating unit within a polymer chain. It is also important to be able to generate the full polymer structure from a repeating unit. Enclosed within the bracket can be one repeating unit (equivalent of a monomer), or two, or three, etc. Let’s use polyvinylidene chloride as an example:
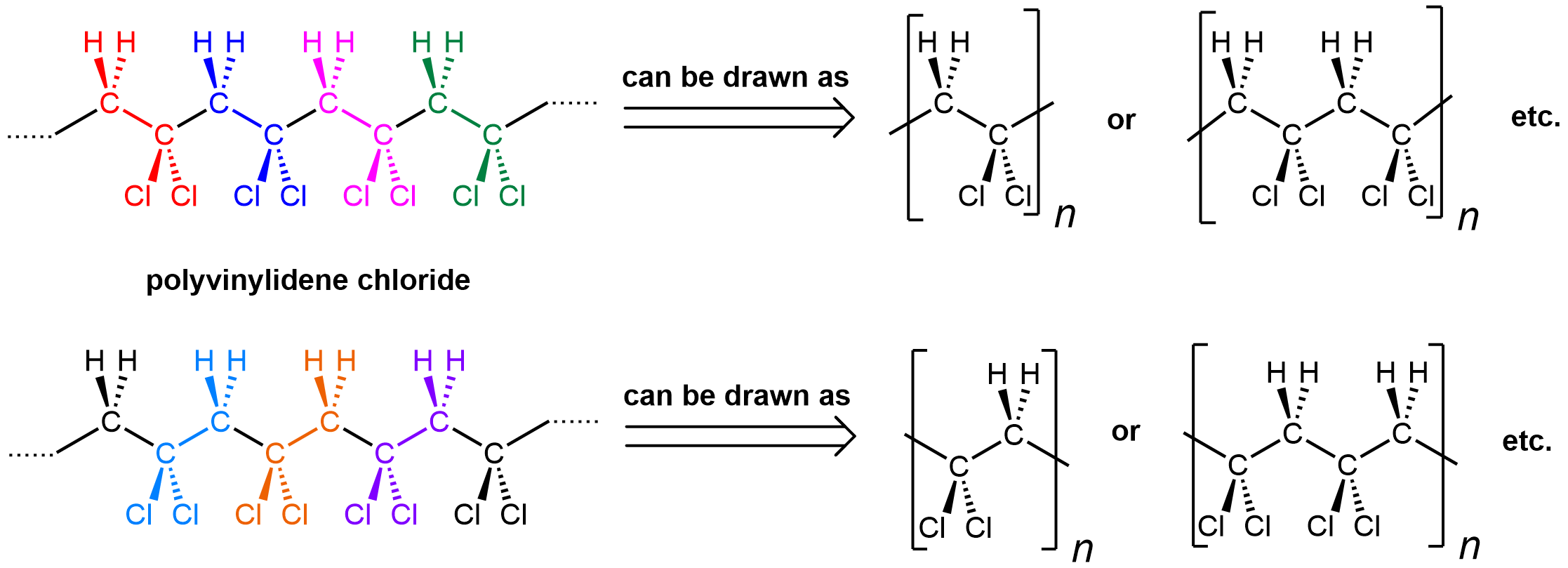
Shown on the left is a polyvinylidene chloride polymer strand. The individual repeating units are color-coded. However, there are two ways to consider its repeating units: starting at the CH2 (top) or the CCl2 (bottom). Either way is fine.
You can see that the repeating pattern in this polymer is alternating CH2 and CCl2. When you put a bracket around a repeating unit, you are communicating that what is outside of the bracket exactly repeats what is inside, starting immediately with the bonds that are divided by the bracket. Therefore, the leftmost carbon atom of the next repeating unit is directly bonded to the rightmost carbon of the unit shown.
In other words, something like the following is incorrect for depicting polyvinylidene chloride:
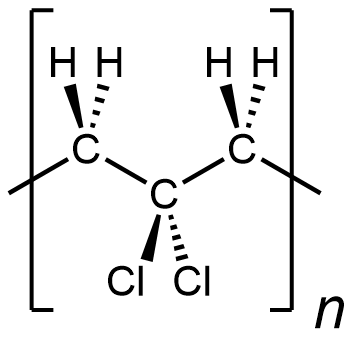
It is depicting a polymer where there are CH2 unit bonded to CH2 unit, which does not occur in polyvinylidene chloride. (This polymer would be …-CH2-CH2-CCl2-CH2-CH2-CCl2-CH2-CH2-CCl2-…)
Exercise: Identifying a Monomer from a Polymer Structure
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

