D14.7 Haber-Bosch Process
The interplay between thermodynamics and kinetics is illustrated in the industrial synthesis of ammonia. It became possible to manufacture ammonia in useful quantities in the early 20th century after the factors that influence this reaction were understood:
Each year, ammonia is among the top 10 (by mass) chemicals manufactured in the world. It plays a vital role in the global economy. It is used in the production of fertilizers and is itself an important fertilizer for enhancing growth of corn, cotton, and other crops. Large quantities of ammonia are converted to nitric acid, which plays an important role in the production of fertilizers, explosives, plastics, dyes, and fibers, and is also used in the steel industry.
The overall forward reaction is exothermic. Estimating via average bond energies: the six new N-H bonds formed would release 6 × 391 kJ/mol, while the N≡N bond broken requires 946 kJ/mol and the three H-H bonds broken requires 3 × 436 kJ/mol. (ΔrH° = [946 kJ/mol + 3×436 kJ/mol] – 6×391 kJ/mol = -92 kJ/mol). Calculating using standard formation enthalpies, ΔfH°, also results in ΔrH° = -92 kJ/mol.
The overall forward reaction results in a decrease in entropy: 4 moles of gaseous substances are transformed to 2 moles of gaseous substances. Calculating using standard entropy values gives ΔrS° = -199 J/K·mol.
Therefore, thermodynamics of this reaction indicates that the overall reaction is product favored at 25°C, ΔrG° = -33 kJ/mol. But the activation-energy barrier for the forward reaction is significant: an N≡N bond and three H-H bonds need to be broken for this reaction to proceed. One proposed mechanism for this reaction is:
| step 1: | N2(g) + H2(g) | ⟶ | NNH2(g) | Ea,1 = 524 kJ/mol |
| step 2: | NNH2(g) + H2(g) | ⟶ | H2NNH2(g) | Ea,2 = 128 kJ/mol |
| step 3: | H2NNH2(g) | ⟶ | HNNH3(g) | Ea,3 = 253 kJ/mol |
| step 4: | HNNH3(g) + H2(g) | ⟶ | 2NH3(g) | Ea.4 = 103 kJ/mol |
Step 1, with the largest Ea, is the rate-determining step. This barrier is so large that no reaction would take place at room temperature (80% of our air is comprised of N2; it is a very stable (unreactive) molecule). Raising the reaction temperature is necessary to speed it up. However, the reaction crosses over to being reactant-favored at temperatures above 190 °C, and given the size of Ea,1, we would need to heat to significantly higher than 190 °C to achieve any reasonable rate.
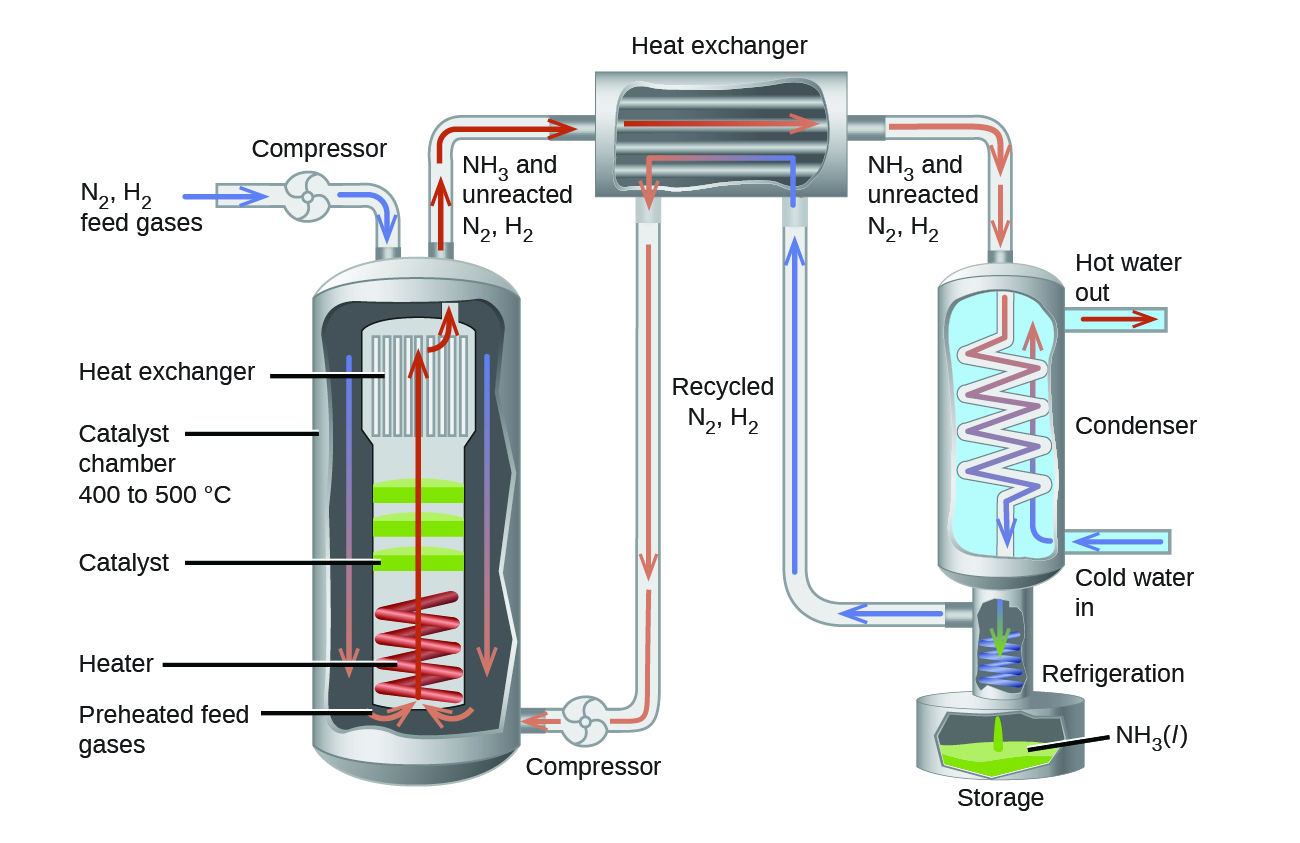
With all the thermodynamics and kinetics factors in mind, the Haber-Bosch process tackled this reaction on several fronts to make it into a practical industrial process that gives a large yield of product relatively quickly.
Increasing the pressure on the system shifts the reaction equilibrium toward the product side, increasing the concentration and partial pressure of ammonia. A heterogeneous catalyst is used to increase the rate of the reaction to allow operations at reasonable temperatures. Finally, taking advantage of the fact that ammonia has a higher boiling point than nitrogen and hydrogen, the NH3 produced is condensed to a liquid at temperatures where N2 and H2 remain gases to allow efficient extraction of product from the reaction mixture. (Theoretically, NH3 could be condensed in the reaction vessel to remove the product and shift the equilibrium toward the product side, but practically, the reaction operates at temperatures too high for condensing NH3).
Exercise: Intermolecular Forces and Boiling Point
Ammonia has a higher boiling point than nitrogen and hydrogen because of intermolecular forces.
In the commercial production of ammonia, reaction conditions are typically 400 – 500 °C and 150–250 bar. The catalyst consists of Fe3O4 mixed with KOH, Al2O3, and SiO2. This gives the best compromise among rate, product yield, and the cost of the equipment necessary to produce and contain high-pressure gases at high temperatures.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

