D14.6 Heterogeneous Catalysts
A heterogeneous catalyst is present in a different phase from the reactants. Such catalysts are usually solids, and often function by furnishing an active surface upon which one or more steps in the reaction can occur.
A heterogeneous catalytic reaction has at least four steps in its reaction mechanism:
- Adsorption of the reactant(s) onto the surface of the catalyst
- Activation of the adsorbed reactant(s)
- Reaction of the adsorbed reactant(s)
- Diffusion of the product(s) from the surface into the gas or liquid phase (desorption)
Any one of these steps may be slow and thus may serve as the rate determining step. But the overall rate of the reaction is still faster than it would be without the catalyst. The figure below illustrates catalysis the reaction of an alkene with hydrogen on the surface of a nickel catalyst. The overall reaction is
![]()
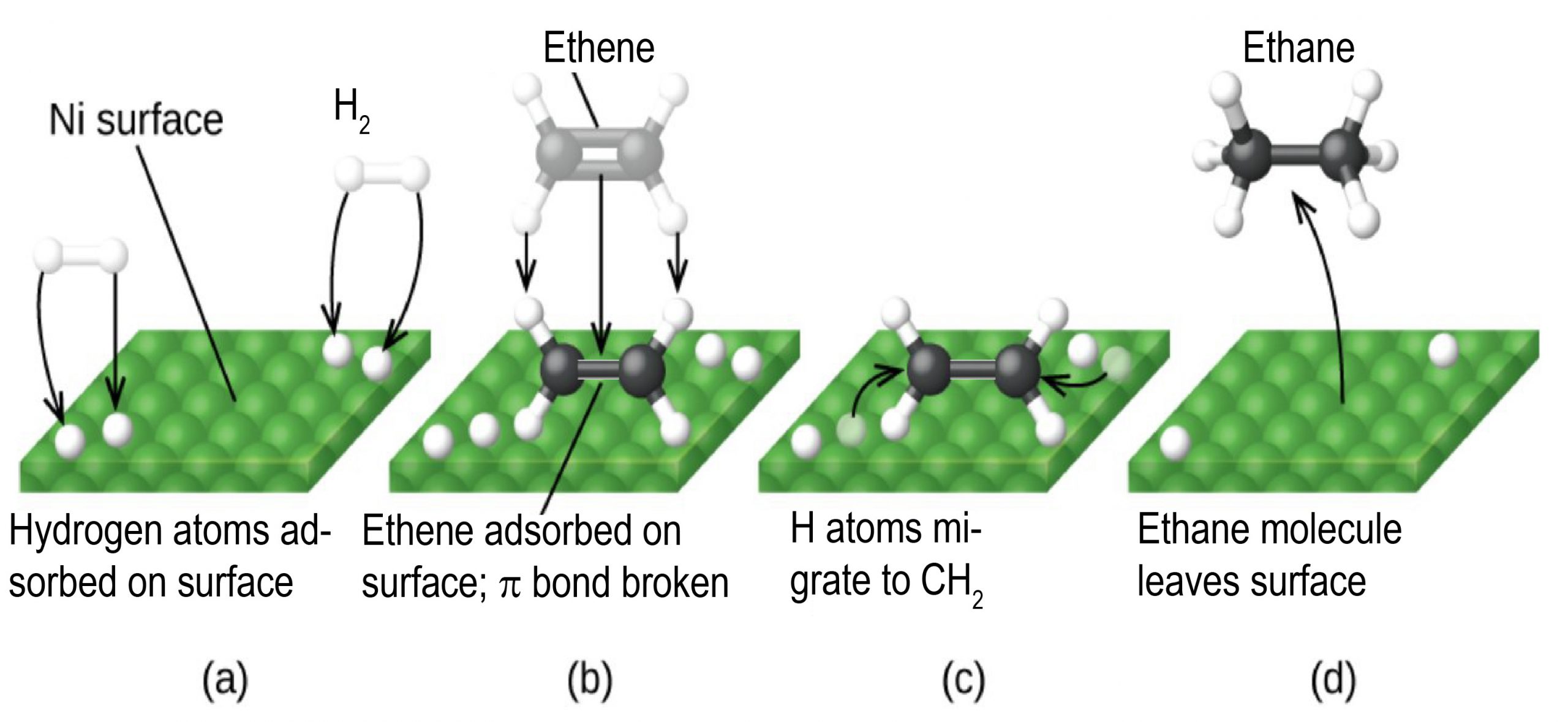
The uncatalyzed C2H4(g) + H2(g) ⟶ C2H6(g) reaction would necessitate a transition state where the C=C π bond and the H-H σ bond are breaking while the two C-H σ bonds form. Such a transition state is so high in energy that without a catalyst, H2 is unreactive towards alkenes under most conditions.
Nickel is a catalyst often used in the hydrogenation of polyunsaturated fats and oils to produce saturated fats and oils. Other significant industrial processes that involve the use of heterogeneous catalysts include the preparation of sulfuric acid, the synthesis of ammonia from nitrogen and hydrogen, the oxidation of ammonia to nitric acid, and the synthesis of methanol. Heterogeneous catalysts are also used in the catalytic converters found on most gasoline-powered automobiles.
Exercise: Catalysis and Reaction Energy Diagram
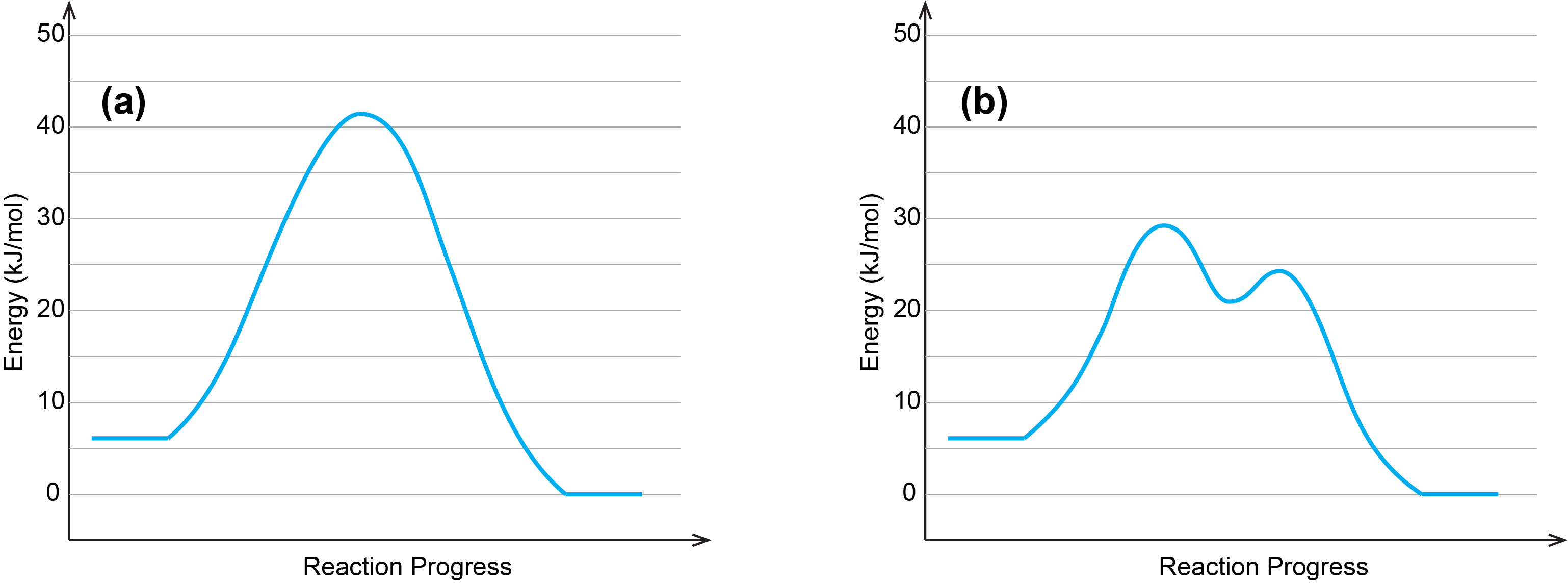
One of the two reaction energy diagrams above corresponds to a reaction occurring without a catalyst, the other corresponds to a catalyzed reaction. Identify which diagram corresponds to the reaction occurring with a catalyst. In your notebook, explain why you chose that diagram and determine the activation energy for the catalyzed reaction.
Write in your notebook, then left-click here for an explanation.
A catalyst does not affect the energy of reactants or products, so those aspects of both diagrams are the same. There are two major differences between the graphs:
- Graph (b) has two transition states while graph (a) has one transition state;
- The highest energy transition state in graph (b) is significantly lower in energy than the transition state in graph (a).
These indicate the use of a catalyst in (b), as it is depicting a multi-step reaction with a lower activation energy.
There are two activation energies in (b).
- Ea,1 is the difference between the energy of the starting reagents and the first transition state. In (b) the reactants are at 6 kJ/mol and the first transition state is at 29 kJ/mol, so Ea,1 is: 29 kJ/mol – 6 kJ/mol = 23 kJ/mol.
- Ea,2 is the difference between the energy of the intermediates and the second transition state. In (b) the intermediates are at 21 kJ/mol and the second transition state is at 24 kJ/mol, so Ea,2 is 24 kJ/mol – 21 kJ/mol = 3 kJ/mol.
Exercise: Catalytic Mechanisms
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

