D23.5 Secondary Batteries
Secondary batteries are rechargeable; that is, the reaction that powers the battery can be reversed so that the original reactants can be regenerated. Secondary batteries are found in smartphones, electronic tablets, automobiles, and many other devices.
Lead-Acid Battery
Lead-acid battery is the type of secondary battery used to start gasoline-powered automobiles. It is inexpensive and capable of producing the high current required by the starter motors when starting a car. Lead-acid batteries are heavy because of lead’s high density, they contain highly corrosive concentrated sulfuric acid, and they must be disposed of properly to avoid lead-poisoning hazards. But they can produce a lot of current in a short period of time so for certain applications they are the best choice.
The reactions occurring in a lead acid battery are:
| Oxidation (anode): | Pb(s) + HSO4–(aq) | ⟶ | PbSO4(s) + H+(aq) + 2e– | E°anode = -0.359 V |
| Reduction (cathode): | PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e– | ⟶ | PbSO4(s) + 2H2O(ℓ) | E°cathode = +1.690 V |
| overall: | Pb(s) + PbO2(s) + 2H2SO4(aq) | ⟶ | 2PbSO4(s) + 2H2O(ℓ) | E°cell = +2.049 V |
Each cell produces 2.05 V, so six cells can be connected in series to produce a 12-V car battery.
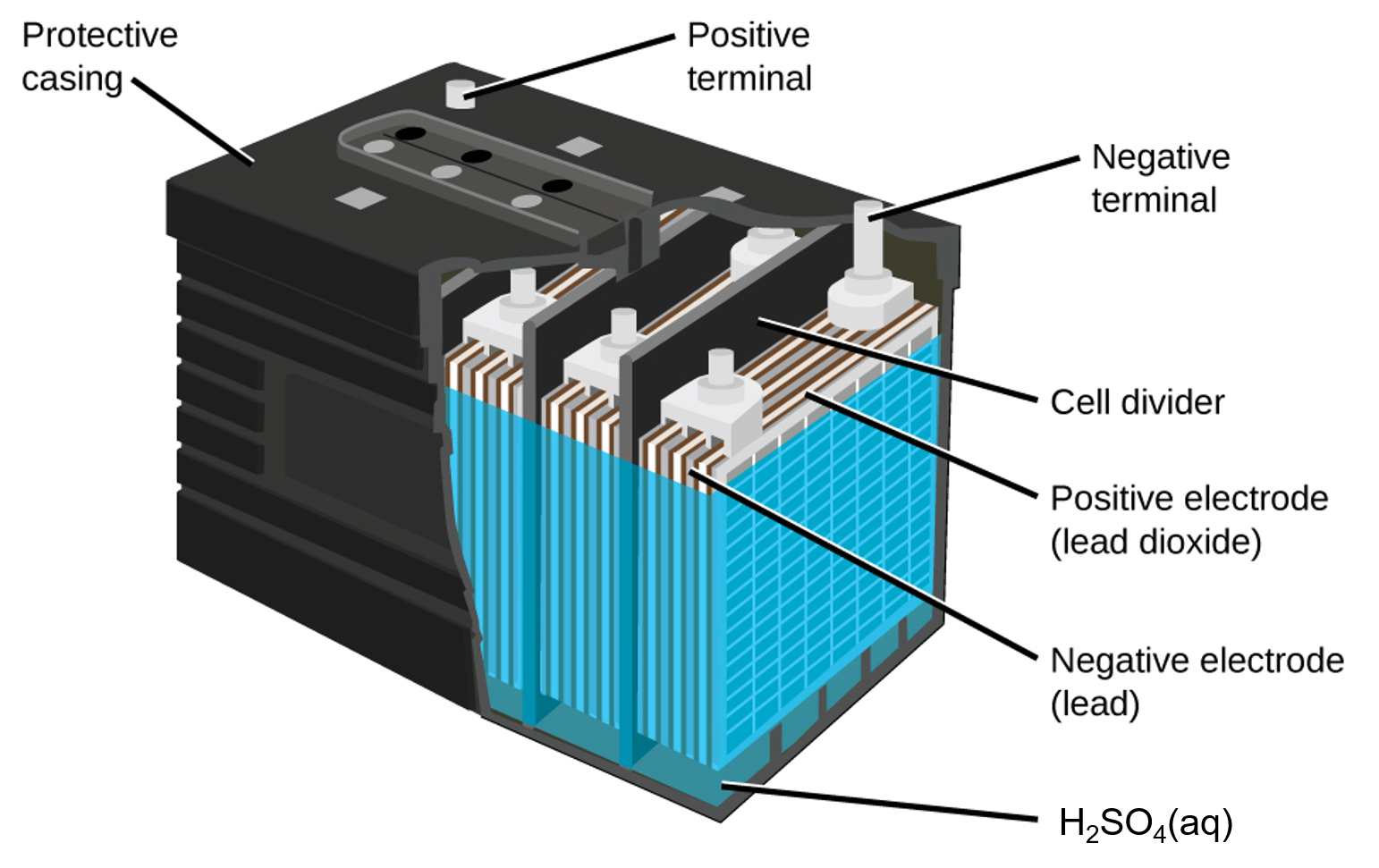
In each cell, the lead electrodes are immersed in sulfuric acid. The anodes are spongy lead metal and the cathodes are lead impregnated with lead oxide. As the battery is discharged, a powder of PbSO4 forms on the electrodes. When a lead-acid battery is recharged by a car’s alternator, electrons are forced to flow in the opposite direction which reverses the reactions at anode and cathode, in other words, the cell undergoes electrolysis reactions to replenish the substances that have reacted away.
Practically, the concentrated sulfuric acid becomes quite viscous when the temperature is low, inhibiting the flow of ions between the plates and reducing the current that can be delivered. This effect is well-known to anyone who has had difficulty starting a car in cold weather. These batteries also tend to slowly self-discharge, so a car left idle for several weeks might be unable to start. And after thousands of discharge-charge cycles, PbSO4 that does not get converted to PbO2 gradually changes to an inert form which limits the battery capacity. Also, “fast” charging causes rapid evolution of potentially explosive H2 gas from the water in the electrolyte (electrolysis of water); the gas bubbles form on the lead surface and can tear PbO2 off the electrodes. Eventually enough solid material accumulates at the bottom of the electrolyte to short-circuit the battery, leading to its permanent demise.
Exercise: Lead-acid Batteries
Lithium Ion Battery
Lithium ion batteries are among the most popular rechargeable batteries and are used in many portable electronic devices because their advantages outweigh the disadvantage of higher cost. In a typical Li-ion battery the reactions are:
| Oxidation (anode): | LiCoO2 | ⟶ | Li1-xCoO2 + x Li+ + x e– |
| Reduction (cathode): | x Li+ + x C6 + x e– | ⟶ | x LiC6 |
| overall: | LiCoO2 + x C6 | ⟶ | Li1-xCoO2 + x LiC6 |
(x is no more than about 0.5.) The battery voltage is about 3.7 V.
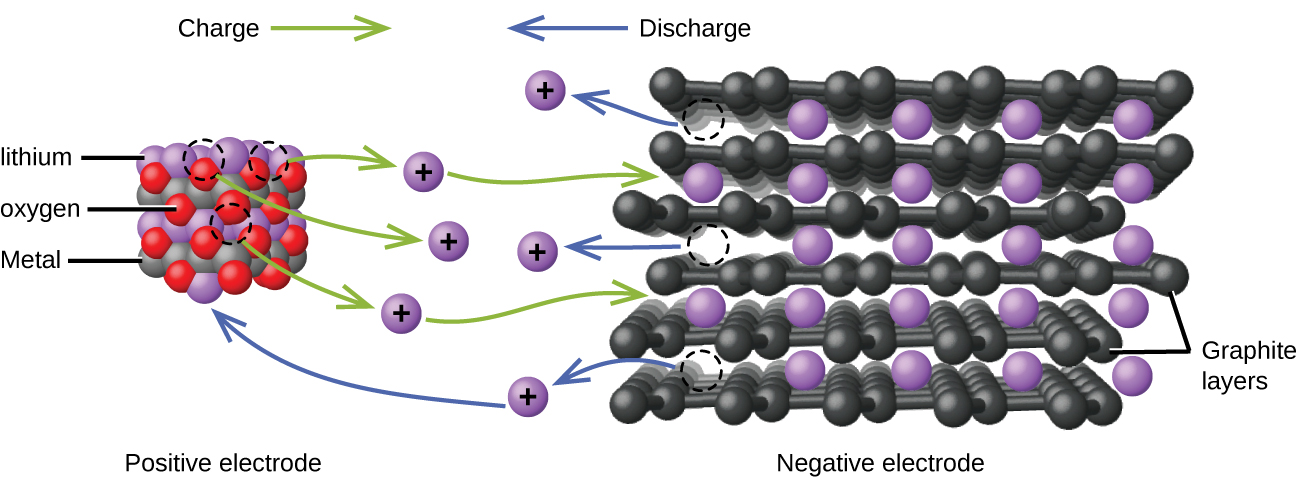
Lithium batteries are popular because they can provide a large amount of current, are lighter than comparable batteries of other types, produce a nearly constant voltage as they discharge, and only slowly lose their charge when stored.
Exercise: Lithium-Ion Batteries
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

