D11.1 Temperature Dependence of Gibbs Free Energy
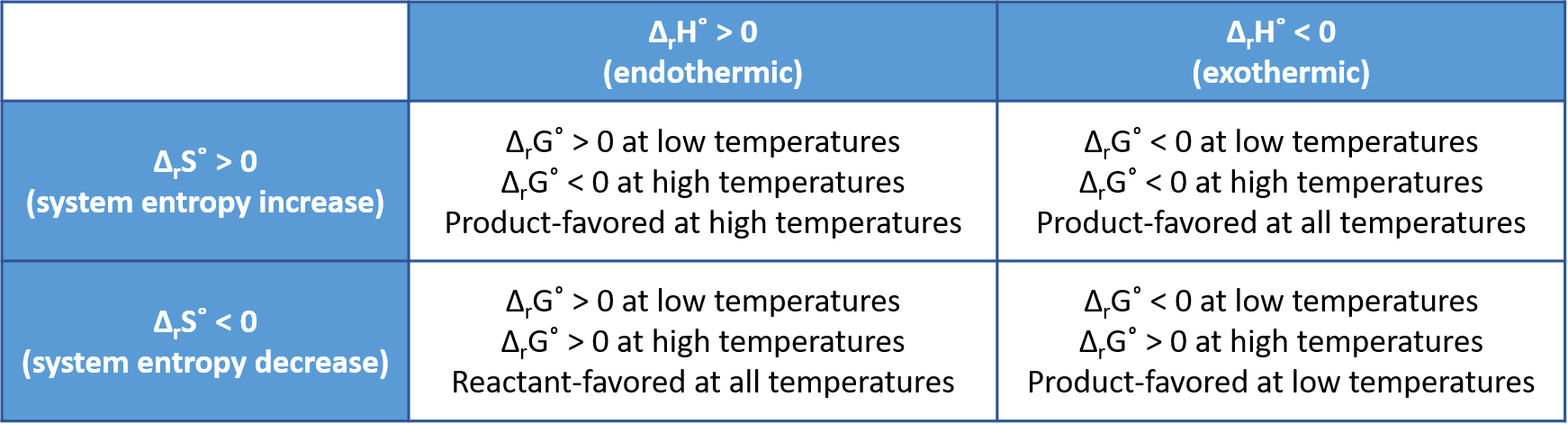
Whether a chemical or physical reaction is product-favored, that is, whether the reactants are converted to products under standard-state conditions, is reflected in the arithmetic sign of its ΔrG°. The equation
shows that the sign of ΔrG° depends on the signs of ΔrH° and ΔrS°, and, in some cases, the absolute temperature (which can only have positive values). For most reactions the values of ΔrH° and ΔrS° change very little as temperature changes, so their signs do not change. Four scenarios are possible:
- Both ΔrH° and ΔrS° are positive—This is an endothermic reaction with an increase in system entropy. ΔrG° is negative if TΔrS° > ΔrH°, and positive if TΔrS° < ΔrH°. Such a reaction is product-favored at high temperatures and reactant-favored at low temperatures.
- Both ΔrH° and ΔrS° are negative—This is an exothermic reaction with a decrease in system entropy. ΔrG° is negative if |TΔrS°| < |ΔrH°| and positive if |TΔrS°| > |ΔrH°|. Such a reaction is product-favored at low temperatures and reactant-favored at high temperatures. (Remember that |TΔrS°| represents the magnitude of TΔrS°, ignoring mathematical sign.)
- ΔrH° is positive and ΔrS° is negative—This is an endothermic reaction with a decrease in system entropy. ΔrG° is positive regardless of the temperature. Such a reaction is reactant-favored at all temperatures.
- ΔrH° is negative and ΔrS° is positive—This is an exothermic reaction with an increase in system entropy. ΔrG° is negative regardless of the temperature. Such a reaction is product-favored at all temperatures.
These four scenarios are summarized in this figure:
Activity: Temperature and Product-favored or Reactant-favored Reactions
The incomplete combustion of carbon is described by this equation:
2C(s) + O2(g) ⇌ 2 CO(g)
Without doing any calculations, write in your notebook an explanation of whether this process is product-favored at low temperatures, high temperatures, all temperatures, or no temperature.
Write in your notebook, then left-click here for an explanation.
The following figure illustrates the four scenarios graphically, where ΔrG° is plotted versus temperature:
| ΔrG° | = | − ΔrS°(T) | + | ΔrH° |
| y | = | m(x) | + | b |
In this course, unless specified otherwise, we will assume that ΔrH° and ΔrS° for a given reaction have the same values at all temperatures. Thus, in the preceding figure, the plots representing ΔrG° are linear because the slope of each plot (−ΔrS°) is the same at all temperatures. The orange (scenario 1) and green (scenario 2) plots cross from product-favored to reactant-favored (cross ΔrG° = 0) at a temperature that is characteristic to each specific reaction. This temperature is represented by the x-intercept, which is the temperature, in Kelvin, for which ΔrG° is zero:
Hence, saying a reaction is product-favored at “high” or “low” temperatures is simply indicating whether the temperature is above or below TΔrG°=0 for that reaction. These relative terms are reaction-specific, that is, what is a “high” temperature for one reaction may very well be a “low” temperature for another reaction.
Exercise: Estimating Boiling Point
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

