31 Acidity and Basicity of Salts (M15Q7)
Learning Objectives
- Determine whether a salt solution will be acidic, basic or neutral.
When determining whether a salt solution will be acidic, basic, or neutral, there are a few simple rules we can use to simplify the process:
- Separate the salt into its cation and its anion, evaluating each ion separately.
- If the cation or anion are conjugates of either a strong acid or a strong base, the ion will be neutral. (For example, Cl–(aq) is a neutral ion since it is the conjugate base of HCl(aq), a strong acid.)
- If between the cation and anion, one is a weak acid and one is neutral, the salt solution will be acidic. If between the cation and anion, one is a weak base and one is neutral, the salt solution will be basic.
- If between the cation and anion, one is a weak acid and one is a weak base, compare the Ka of the weak acid and the Kb of the weak base. Whichever is larger will dictate the property of the salt solution.
The Ionization of Hydrated Metal Ions
If we measure the pH of the solutions of a variety of metal ions we will find that these ions act as weak acids when in solution. The aluminum ion is an example. When aluminum nitrate dissolves in water, the aluminum ion reacts with water to give a hydrated aluminum ion, Al(H2O)63+, dissolved in bulk water. What this means is that the aluminum ion has the strongest interactions with the six closest water molecules (the so-called first solvation shell), even though it does interact with the other water molecules surrounding this Al(H2O)63+ cluster as well:
We frequently see the formula of this ion simply as “Al3+(aq)”, without explicitly noting the six water molecules that are the closest ones to the aluminum ion and just describing the ion as being solvated in water (hydrated). This is similar to the simplification of the formula of the hydronium ion, H3O+ to H+. However, in this case, the hydrated aluminum ion is a weak acid and donates a proton to a water molecule. Thus, the hydration becomes important and we may use formulas that show the extent of hydration:
Additional examples of the first stage in the ionization of hydrated metal ions are:
Example 1
Hydrolysis of [Al(H2O)6]3+
Calculate the pH of a 0.10 M solution of aluminum chloride, which dissolves completely to give the hydrated aluminum ion Al(H2O)63+ in solution.
Solution
In spite of the unusual appearance of the acid, this is a typical acid ionization problem.
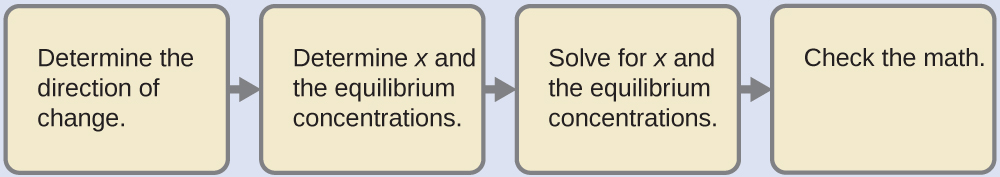
- Determine the direction of change. The equation for the reaction and Ka are:
Al(H2O)63+(aq) + H2O(l) ⇌ H3O+(aq) + Al(H2O)5(OH)2+(aq) Ka = 1.4 × 10-5The reaction shifts to the right to reach equilibrium.
- Determine x and equilibrium concentrations. Use the table:
Al(H2O)63+(aq) + H2O(l) ⇌ H3O+(aq) + Al(H2O)5(OH)2+(aq) I 0.10 0 0 C – x + x + x E 0.10-x x x - Solve for x and the equilibrium concentrations. Substituting the expressions for the equilibrium concentrations into the equation for the ionization constant yields:
Ka = [latex]\frac{[H_{3}O^{+}][Al(H_{2}O)){5}(OH]^{2+}}{[Al(H_{2}O)_{6}^{3+}]}[/latex] = [latex]\frac{(x)(x)}{0.10 -x}[/latex] = 1.4 × 10-5
Solving this equation (assuming 0.10 – x ≈ x) gives:
x = 1.2 × 10-3 MFrom this we find:
[H3O+] = 0 + x = 1.2 × 10-3 MpH = -log[H3O+] = 2.92 (an acidic solution) - Check the work. The arithmetic checks; when 1.2 × 10−3 M is substituted for x, the result = Ka.
Check Your Learning
What is [Al(H2O)5(OH)]2+ in a 0.15 M solution of Al(NO3)3 that contains enough of the strong acid HNO3 to bring [H3O+] to 0.10 M?
Answer:
2.1 × 10−5 M
The constants for the different stages of ionization are not known for many metal ions, so we cannot calculate the extent of their ionization. However, practically all hydrated metal ions other than those of the alkali metals ionize to give acidic solutions. Ionization increases as the charge of the metal ion increases or as the size of the metal ion decreases.
Key Concepts and Summary
Ionic compounds can also create acidic or basic solutions when dissolved in water. First, separate the ionic compound into its cation and anion and evaluate each ion separately as a weak acid, weak base, or neutral ion. The conjugate acids/bases of strong acids/bases are neutral. When you bring both ions together, it is sometimes clear that the solution is acidic or basic, but if the cation is acidic and the anion is basic, compare the Ka and the Kb of the cation and anion and whichever is larger dictates the property of the solution. Hydrated metal ions form weak acids in solution.
Chemistry End of Section Exercises
- Write a statement to help another student identify whether a metal ion is acidic, basic or neutral.
- Determine whether the following substances are acidic, basic or neutral when dissolved in water:
- NaCl
- Na2SO4
- NH4Cl
- FeCl3
- CO2
- K2O
- NH4NO2 (Kb of NH3 = 1.8 × 10-5; Ka of HNO2 = 7.4 × 10-4)
Answers to Chemistry End of Section Exercises
- No metal ions are basic. Metal ions from group I, Ca2+, Sr2+, and Ba2+ are considered neutral. The remaining metal ions are acidic.
-
- neutral
- basic
- acidic
- acidic
- acidic
- basic
- acidic


Feedback/Errata