4 Alkenes: Naming, Geometric Isomers, Intermolecular Forces and Bond Properties; Optical Isomers (M12Q3)
Learning Objectives
- Draw structures and write chemical formulas for alkenes when given only the compound’s name.
| Nomenclature | - Draw, recognize, and name geometric isomers (cis/trans) of alkenes and cycloalkanes.
| Geometric Isomers | - Use strength of intermolecular forces in organic molecules to explain differences in physical properties such as boiling point and solubility.
| Intermolecular Forces | - Determine whether a molecule has optical isomers (enantiomers) and identify chiral carbon atoms.
| Optical Isomers |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Alkenes
Organic compounds that contain one or more double or triple bonds between carbon atoms are described as unsaturated. Unsaturated hydrocarbon molecules that contain one or more double bonds are called alkenes. Carbon atoms linked by a double bond are bound together with one σ bond and one π bond.
Ethene, C2H4, is the simplest alkene. Each carbon atom in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene) (Figure 1); butene follows in the series. We will look more closely at butene later, learning about a new form of isomerism.
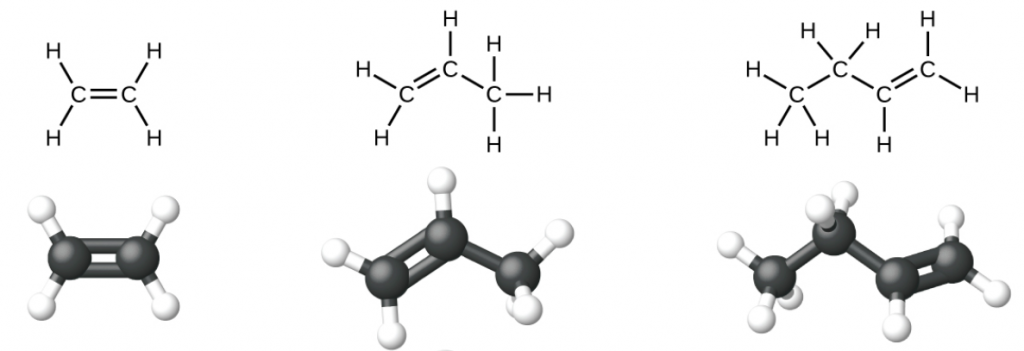
Nomenclature of Alkenes
The name of an alkene is derived from the name of the alkane with the same number of carbon atoms. The presence of the double bond is signified by replacing the suffix -ane with the suffix -ene. The location of the double bond is identified by naming the smaller of the numbers of the carbon atoms participating in the double bond. When assigning numbers, the double bond takes priority over any substituents attached to the longest carbon chain and should be given the lowest possible number assignment. Some alkenes (like 2-butene) will also have a prefix associated with what type of isomer it is, which is described in the next section.
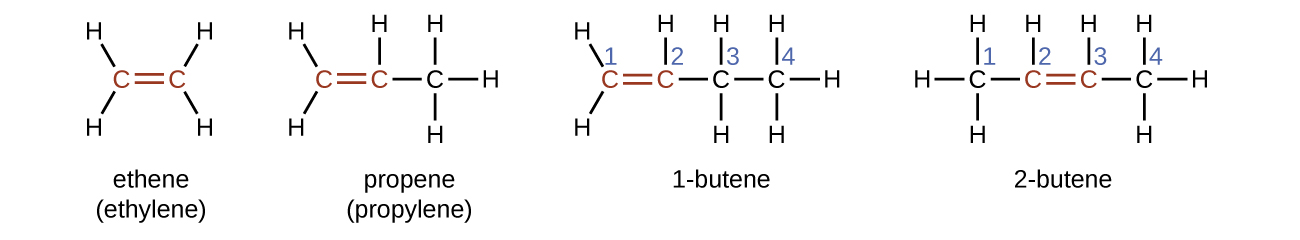
Isomers of Alkenes
1-butene and 2-butene are structural isomers, since they have the same formula but a different arrangement of atoms. For example, the first carbon atom in 1-butene is bonded to two hydrogen atoms, but the first carbon atom in 2-butene is bonded to three hydrogen atoms.
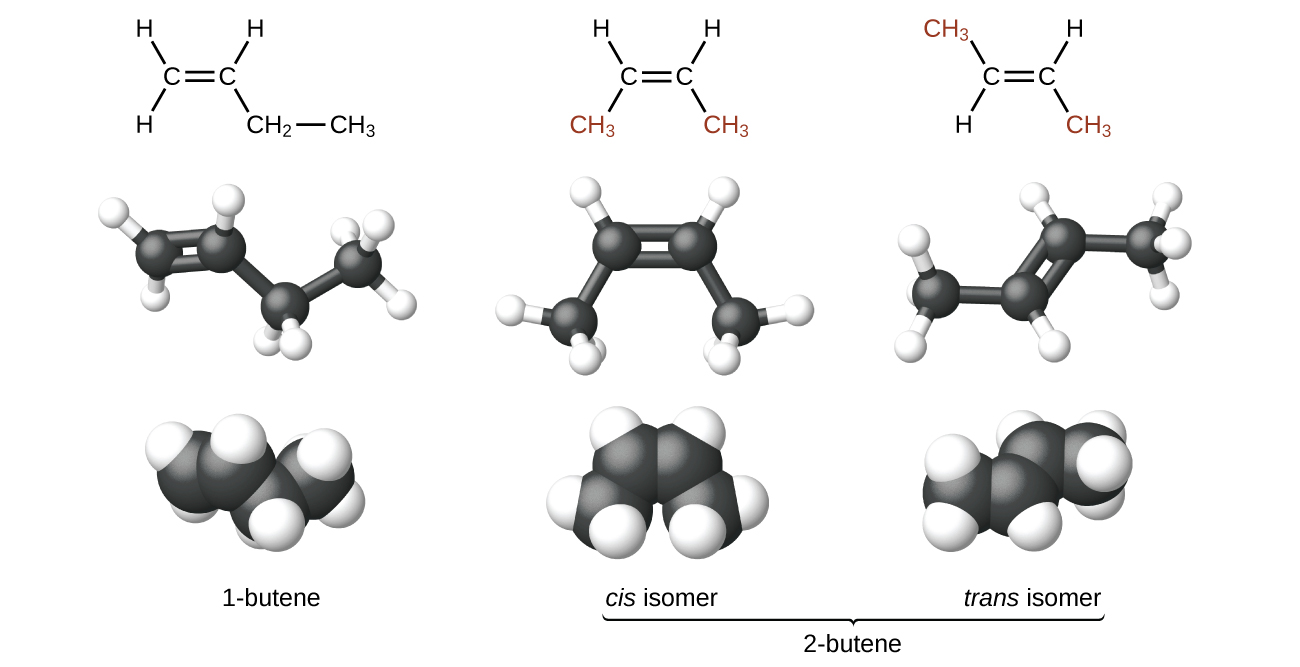
The compound 2-butene and some other alkenes also form a second type of isomer called a geometric isomer. In a pair of geometric isomers, the atoms are attached in the same order, but the spatial arrangement of the two molecules differ. In order to be geometric isomers, each carbon of the C=C must have two different groups attached to it (for example, in 2-butene, each carbon of the C=C is attached to a hydrogen and a methyl group).
Recall that carbon atoms are free to rotate around a single bond, but not around a double bond; a double bond is rigid. This makes it possible to have two isomers of 2-butene, one with both methyl groups on the same side of the double bond (cis-) and one with the methyl groups on opposite sides (trans-) as seen in (Figure 2).
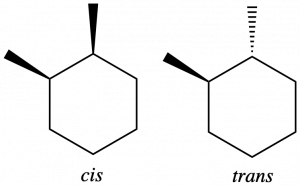
Geometric isomers can also form in cycloalkanes when adjacent carbons have two different bonded groups. In 1,2-dimethylcyclohexane, two carbons in the ring each have a methyl and a hydrogen attached. The methyl groups can both be on the same side of the ring, forming a cis-isomer (see left structure below) or they can be on opposite sides of the ring, forming a trans-isomer (see right structure below).
Intermolecular Forces of Alkenes
The intermolecular forces of alkenes are very similar to that of alkanes. Increasing the length of the longest carbon chain will increase the strength of the dispersion forces. As the molar mass of the molecules increases, the boiling and melting points will also increase. Additionally, the less branched an isomer is, the greater surface area the molecule will have to interact with other molecules, leading to stronger dispersion forces and higher boiling and melting points.
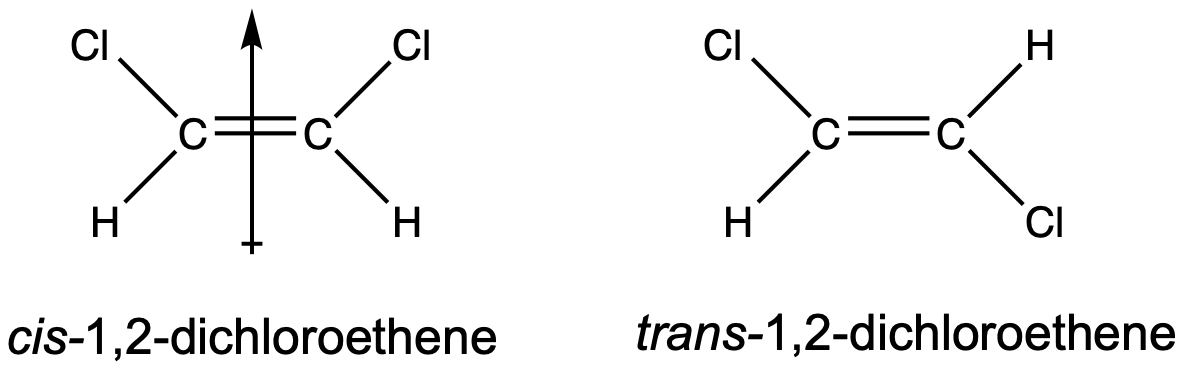
Geometric isomers may have different boiling and melting points, even though they have the same connectivity/branching. When there are polar bonds within cis/trans molecules, often the cis-isomer is more polar than the trans-isomer. A good example is 1,2-dichloroethene, shown in Figure 3. The cis-isomer has a molecular dipole moment but the trans-isomer does not have a molecular dipole moment, since the two C–Cl bond dipoles cancel each other out. Because the cis-isomer exhibits both dispersion and dipole-dipole forces but the trans-isomer only exhibits dispersion forces, the cis-isomer will have significantly higher boiling and melting points.
Optical Isomers and Chirality
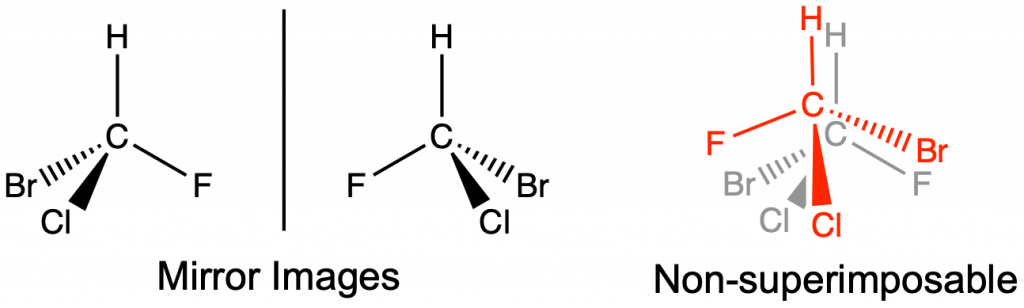
Optical isomers have the same molecular formula and connectivity, but are mirror images of each other and not superimposable. Optical isomers have at least one chiral carbon. A chiral carbon is a sp3 hybridized carbon with four different groups attached (Figure 4). Optical isomers are extremely important in biological applications, such as pharmaceutical development. Optical isomers share the same intermolecular forces and thus have equivalent boiling and melting points. Separation of optical isomers is still possible, but requires special equipment and techniques that are beyond the scope of this text.
Chemistry in Real Life: Recycling Plastics
Polymers (from Greek words poly meaning “many” and mer meaning “parts”) are large molecules made up of repeating units, referred to as monomers. Polymers can be natural (starch is a polymer of sugar residues and proteins are polymers of amino acids) or synthetic [like polyethylene, polyvinyl chloride (PVC), and polystyrene]. The variety of structures of polymers translates into a broad range of properties and uses that make them integral parts of our everyday lives. Adding functional groups to the structure of a polymer can result in significantly different properties (see the discussion about Kevlar later in this chapter).
Ethylene (the common industrial name for ethene) is a basic raw material in the production of polyethylene and other important compounds. Over 135 million tons of ethylene were produced worldwide in 2010 for use in the polymer, petrochemical, and plastic industries. Ethylene is produced industrially in a process called cracking, in which the long hydrocarbon chains in a petroleum mixture are broken into smaller molecules.
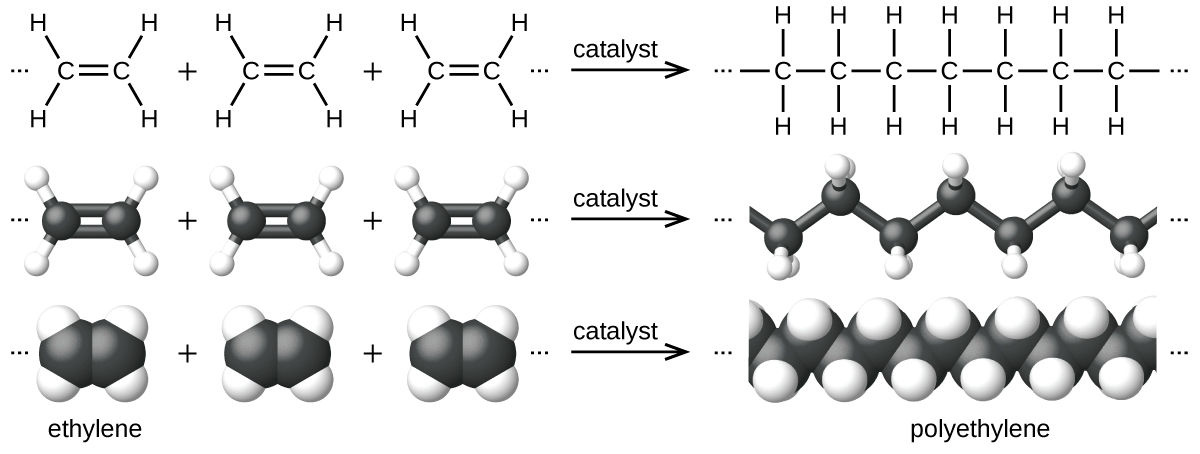
An example of a polymerization reaction is shown in Figure 5. The monomer ethylene (C2H4) is a gas at room temperature, but when polymerized, using a transition metal catalyst, it is transformed into a solid material made up of long chains of –CH2– units called polyethylene. Polyethylene is a commodity plastic used primarily for packaging (bags and films).
Polyethylene is a member of one subset of synthetic polymers classified as plastics. Plastics are synthetic organic solids that can be molded; they are typically organic polymers with high molecular masses. Most of the monomers that go into common plastics (ethylene, propylene, vinyl chloride, styrene, and ethylene terephthalate) are derived from petrochemicals and are not very biodegradable, making them candidate materials for recycling. Recycling plastics helps minimize the need for using more of the petrochemical supplies and also minimizes the environmental damage caused by throwing away these nonbiodegradable materials.
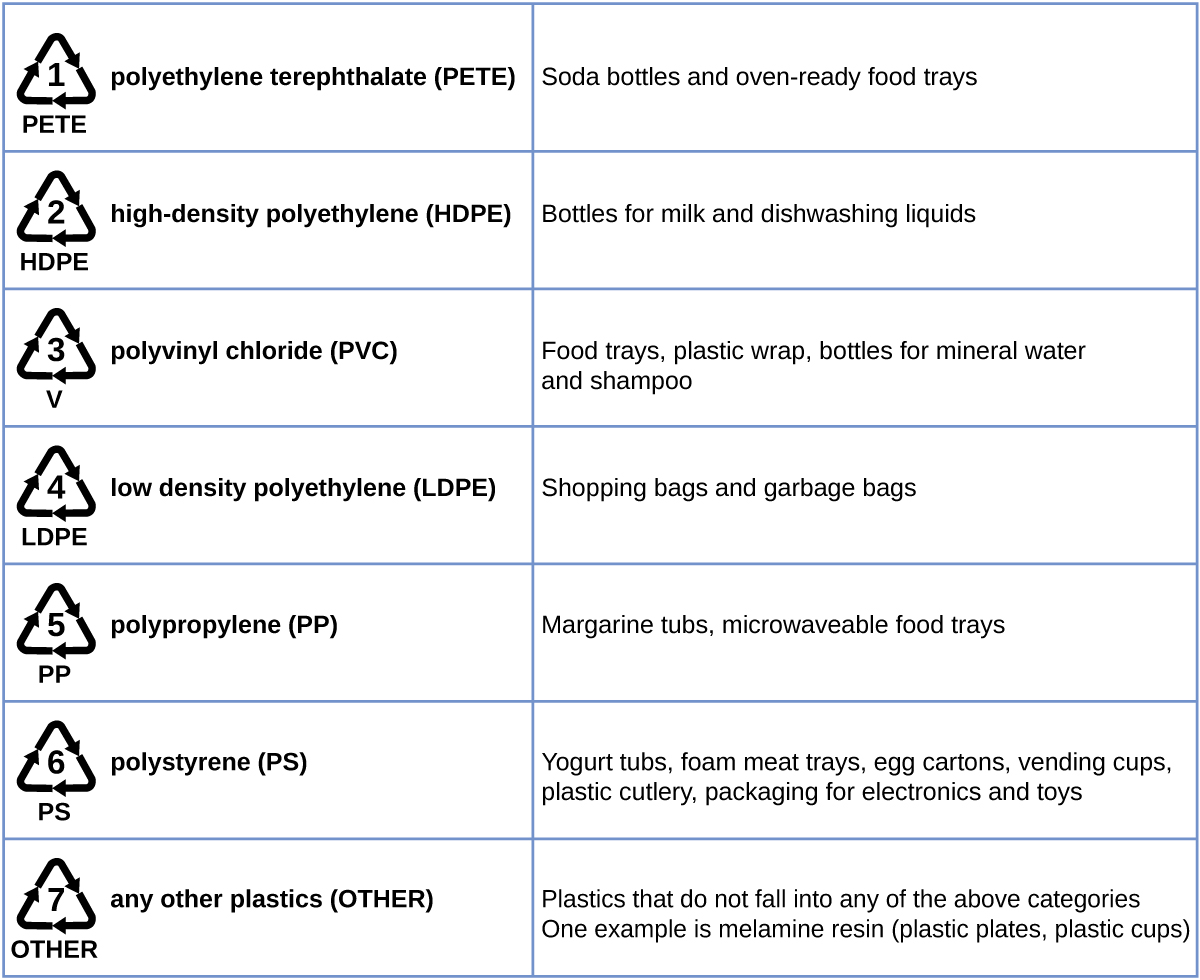
Plastic recycling is the process of recovering waste, scrap, or used plastics, and reprocessing the material into useful products. For example, polyethylene terephthalate (soft drink bottles) can be melted down and used for plastic furniture, in carpets, or for other applications. Other plastics, like polyethylene (bags) and polypropylene (cups, plastic food containers), can be recycled or reprocessed to be used again. Many areas of the country have recycling programs that focus on one or more of the commodity plastics that have been assigned a recycling code (see Figure 6). These operations have been in effect since the 1970s and have made the production of some plastics among the most efficient industrial operations today.
Key Concepts and Summary
Alkenes are unsaturated molecules that contain at least one double bond. The nomenclature of alkenes is very similar to alkanes, except that the double bond is always given the smallest number in the longest carbon chain and the ending changes to –ene from –ane. Alkenes have the potential to form geometric isomers, where two molecules with the same molecular formula and connectivity, but have different spatial arrangements (and often different physical properties).
Although alkenes do not form optical isomers, optical isomers are similar to a geometric isomer in that they have the same molecular formula and connectivity, but they are mirror images of each other. Optical isomers always have a chiral center, or a sp3 hybridized carbon with four different groups attached. It is important to look more holistically at these four groups. For example, all four of these groups could be alkyl chains which begin with carbon. The difference is the length of the alkyl group rather than the fact that each begin with a carbon atom.
Glossary
alkene
a molecule that contains one or more double bond
chiral carbon
a sp3 hybridized carbon with four different groups attached
geometric isomer
molecules that have the same molecular formula and connectivity, but the spatial arrangement of the two molecules differs. These are most often present in molecules with a C=C or cycloalkanes.
optical isomers
molecules that have the same molecular formula and connectivity, but are mirror images of each other caused by a chiral center and are not superimposable
unsaturated
a molecule that contains one or more double or triple bonds between carbon atoms
Chemistry End of Section Exercises
- What is the difference between a saturated and an unsaturated hydrocarbon?
- What is the hybridization of the carbon atoms’ valence orbitals in ethane and ethene?
- Explain why the carbon-carbon bond in ethane rotates freely, while the carbon-carbon bond in ethene does not.
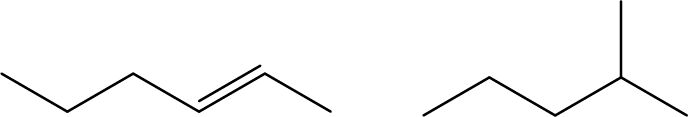
- Explain why these two molecules are not isomers:
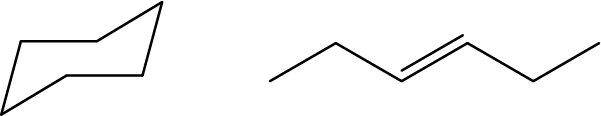
- Are these two molecules isomers? Explain why or why not:
- Write the Lewis structure and molecular formula for each of the following hydrocarbons:
- hexane
- 3-methylpentane
- cis-3-hexene
- 4-methyl-1-pentene
- Write Lewis structures for the cis and trans isomers of .
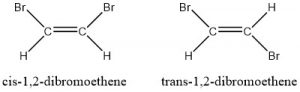
- Consider the two isomers of 1,2-dibromoethene. Which one will have the higher boiling point?
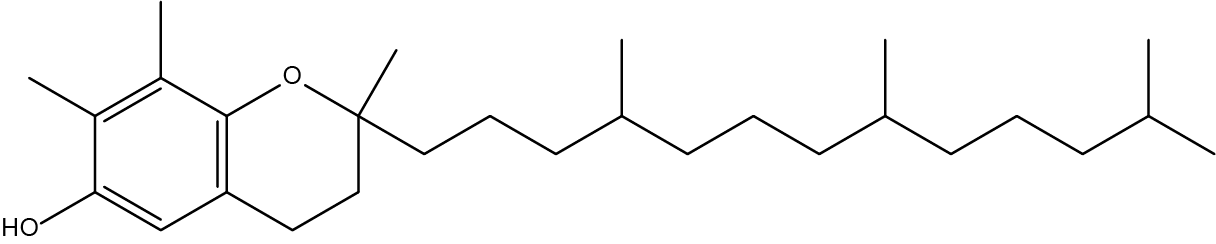
- How many chiral carbons can be found in Vitamin E:
Answers to Chemistry End of Section Exercises
- A saturated hydrocarbon is a molecule containing only single bonds between carbon atoms. An unsaturated hydrocarbon is a molecule with at least one multiple bond (double or triple) between carbon atoms.
- Ethane has a single bond between the two carbons, with three hydrogens bonded to each carbon, resulting in four electron domains and sp3 hybridization. Ethene has a double bond between the two carbons, with two hydrogens bonded to each carbon, resulting in three electron domains and sp2 hybridization.
- The carbon-carbon bond in ethane is a single bond, consisting of a freely-rotating σ bond. The carbon-carbon bond in ethene is a double bond, containing both a σ and π bond. Rotations around the carbon-carbon bond requires breaking and reforming the π bond. It is this lack of free rotation that can give rise to geometric isomers in alkenes.
- The molecules do not have the same molecular formula and so are different molecules, not isomers. The first molecule is C6H12 and the second is C6H14.
- Even though the first molecule is a cycloalkane and the second is an alkene, they are structural isomers because they each have the chemical formula C6H12.
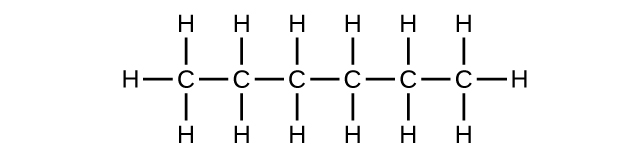
-
- C6H14
- C6H14
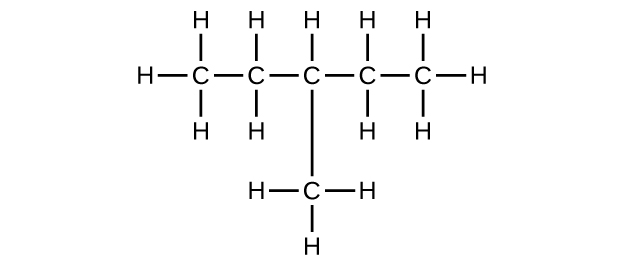
- C6H12
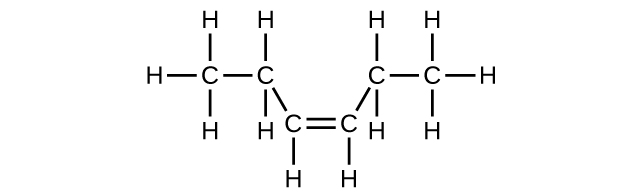
- C6H12
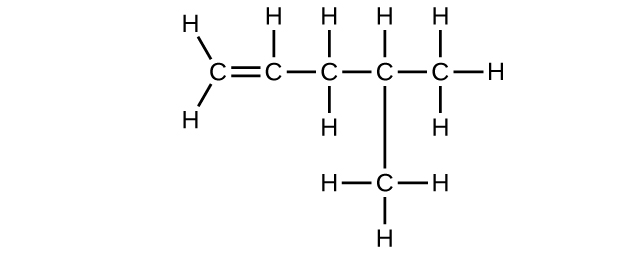
- C6H14
- The cis isomer will have the CH3 and Cl groups oriented along the same side of the π bond. The trans isomer will have the CH3 and Cl groups oriented on the same side of the π bond.
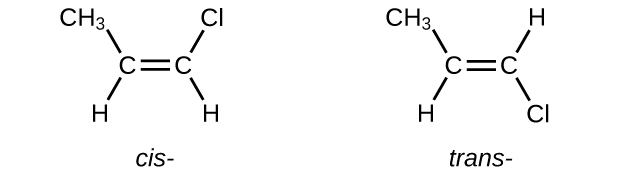
- The cis-1,2-dibromoethene is a polar molecule and the trans-1,2-dibromoethene is a nonpolar molecule. The polar isomer has dipole-dipole intermolecular forces, which are stronger than the dispersion forces of the trans-isomer. The polar isomer’s stronger intermolecular forces gives the cis-isomer a higher boiling point.
- 3


Feedback/Errata