53 Batteries and Fuel Cells (M18Q7)
Learning Objectives
- Describe the components and operation of some common batteries and fuel cells.
| Primary Battery | Secondary Battery | Fuel Cell |
| Key Concepts and Summary | Glossary | End of Section Exercises |
A battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used as a battery. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Real batteries strike a balance between ideal characteristics and practical limitations. For example, the mass of a car battery is about 18 kg or about 1% of the mass of an average car or light-duty truck. This type of battery would supply nearly unlimited energy if used in a smartphone, but would be rejected for this application because of its mass. Thus, no single battery is “best” and batteries are selected for a particular application, keeping things like the mass of the battery, its cost, reliability, and current capacity in mind. There are two basic types of batteries: primary and secondary. A few batteries of each type are described next.

Visit this site to learn more about batteries.
Primary Batteries
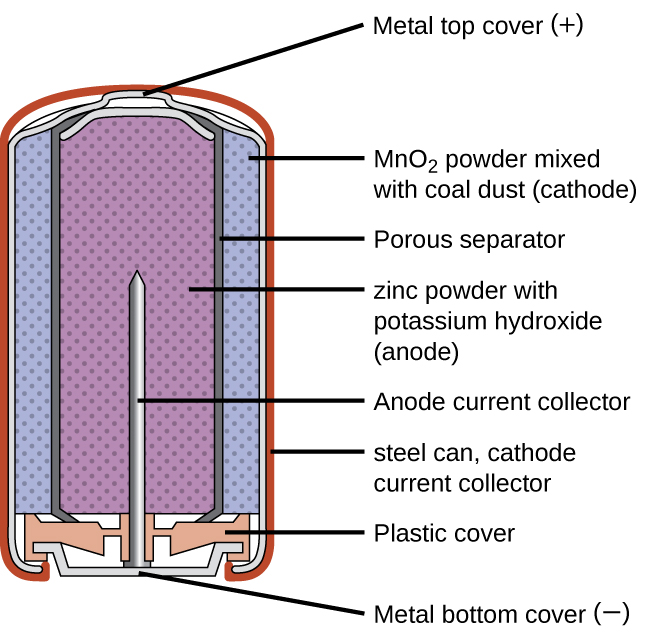
Primary batteries are single-use batteries because they cannot be recharged. A common primary battery type is an alkaline battery (Figure 1) developed in the 1950s. As their name suggests, these types of batteries use alkaline electrolytes, often potassium hydroxide. The reactions are:
| Anode: | Zn(s) + 2 OH–(aq) → ZnO(s) + H2O(l) + 2 e– |
| Cathode: | 2 MnO2(s) + H2O(l) + 2 e– → Mn2O3(s) + 2 OH–(aq) |
| Overall: | Zn(s) + 2 MnO2(s) → ZnO(s) + Mn2O3(s) |
While some alkaline batteries are rechargeable, most are not. Attempts to recharge an alkaline battery that is not rechargeable often leads to rupture of the battery and leakage of the potassium hydroxide electrolyte.

Visit this site to learn more about alkaline batteries.
Secondary Batteries
Secondary batteries are rechargeable. These are the types of batteries found in devices such as smartphones, electronic tablets, and automobiles.
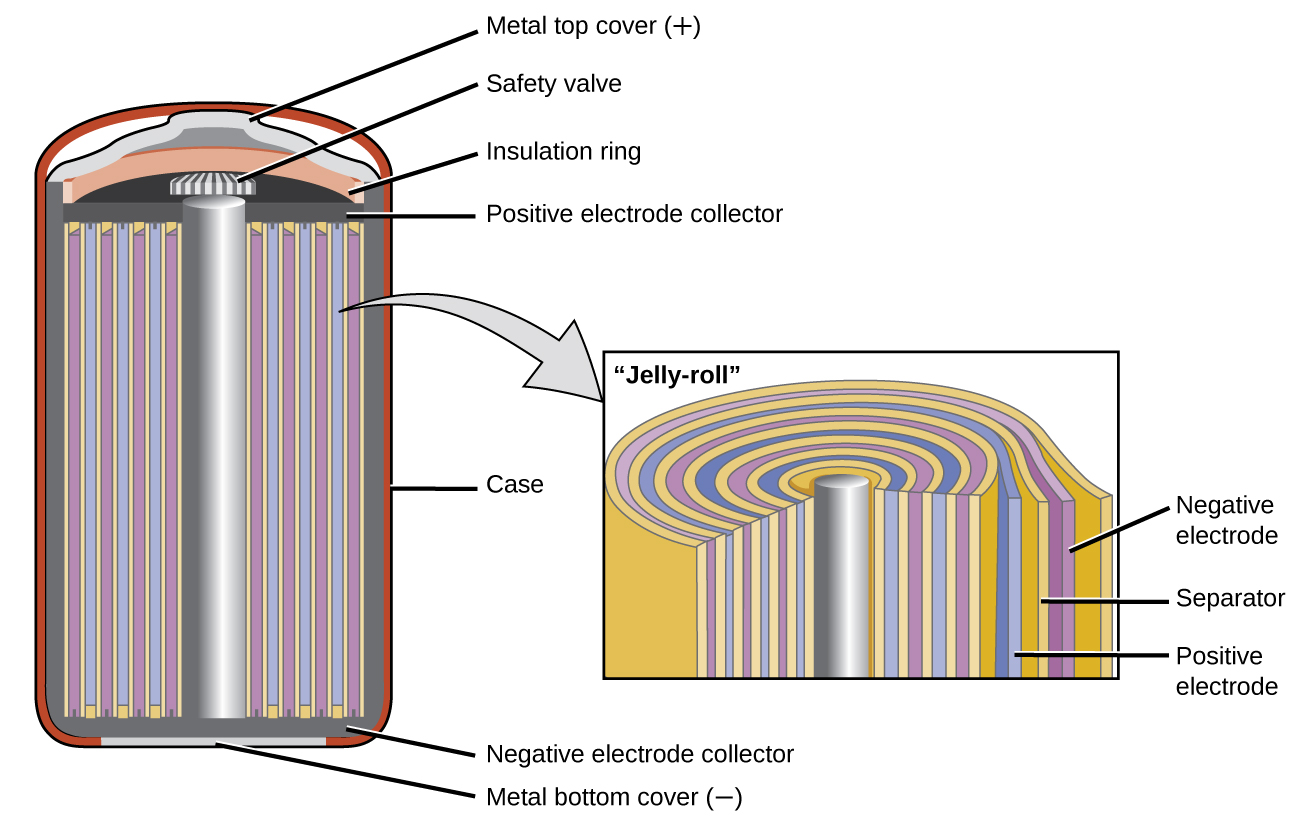
Nickel-cadmium, or NiCd, batteries (Figure 2) consist of a nickel-plated cathode, cadmium-plated anode, and a potassium hydroxide electrode. The positive and negative plates, which are prevented from direct connection by the separator, are rolled together and put into the case. This is a “jelly-roll” design and allows the NiCd cell to deliver much more current than a similar-sized alkaline battery. The reactions are:
| Anode: | Cd(s) + 2 OH–(aq) → Cd(OH)2(s) + 2 e– |
| Cathode: | NiO2(s) + 2 H2O(l) + 2 e– → Ni(OH)2(s) + 2 OH–(aq) |
| Overall: | Cd(s) + NiO2(s) + 2 H2O(l) → Cd(OH)2(s) + Ni(OH)2(s) |
The voltage is about 1.2 V to 1.25 V as the battery discharges. When properly treated, a NiCd battery can be recharged about 1000 times. Cadmium is a toxic heavy metal so NiCd batteries should never be opened or put into the regular trash.

Visit this site for more information about nickel cadmium rechargeable batteries.
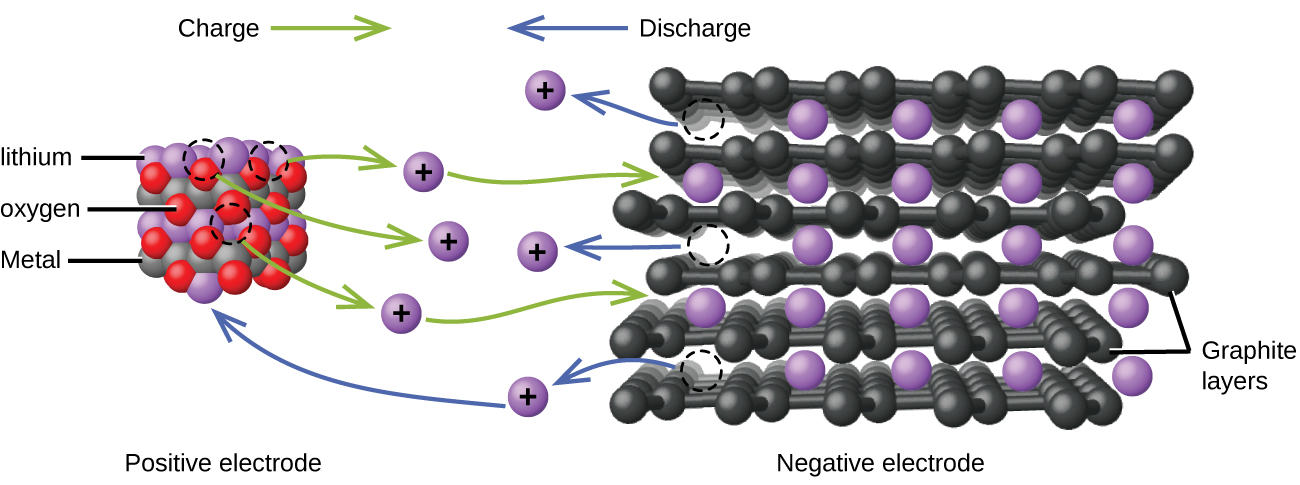
Lithium ion batteries (Figure 3) are among the most popular rechargeable batteries and are used in many portable electronic devices. The reactions are:
| Anode: | LiCoO2 ⇌ Lix-1CoO2 + x Li+ + x e– |
| Cathode: | x Li+ + x e– + x C6 ⇌ x LiC6 |
| Overall: | LiCoO2 + x C6 ⇌ Lix-1CoO2 + x LiC6 |
With the coefficients representing moles, x is no more than about 0.5 moles. The battery voltage is about 3.7 V. Lithium batteries are popular because they can provide a large amount current, are lighter than comparable batteries of other types, produce a nearly constant voltage as they discharge, and only slowly lose their charge when stored.

Visit this site for more information about lithium ion batteries.
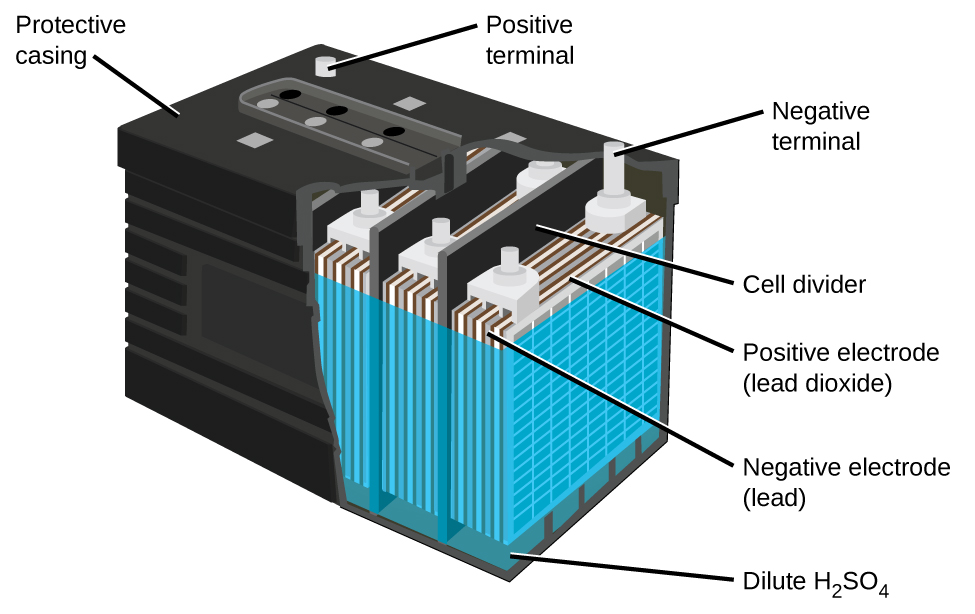
The lead acid battery (Figure 4) is the type of secondary battery used in your automobile. It is inexpensive and capable of producing the high current required by automobile starter motors. The reactions for a lead acid battery are:
| Anode: | Pb(s) + HSO4–(aq) → PbSO4(s) + H+(aq) + 2 e– |
| Cathode: | PbO2(s) + HSO4–(aq) + 3 H+(aq) + 2 e– → PbSO4(s) + 2 H2O(l) |
| Overall: | Pb(s) + PbO2(s) + 2 H2SO4(aq) → 2 PbSO4(s) + 2 H2O(l) |
Each cell produces 2 V, so six cells are connected in series to produce a 12 V car battery. Lead acid batteries are heavy and contain a caustic liquid electrolyte, but are often still the battery of choice because of their high current density. Since these batteries contain a significant amount of lead, they must always be disposed of properly.

Visit this site for more information about lead acid batteries.
Fuel Cells
A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are similar to batteries but require a continuous source of fuel, often hydrogen. They will continue to produce electricity as long as fuel is available. Hydrogen fuel cells have been used to supply power for satellites, space capsules, automobiles, boats, and submarines (Figure 5).
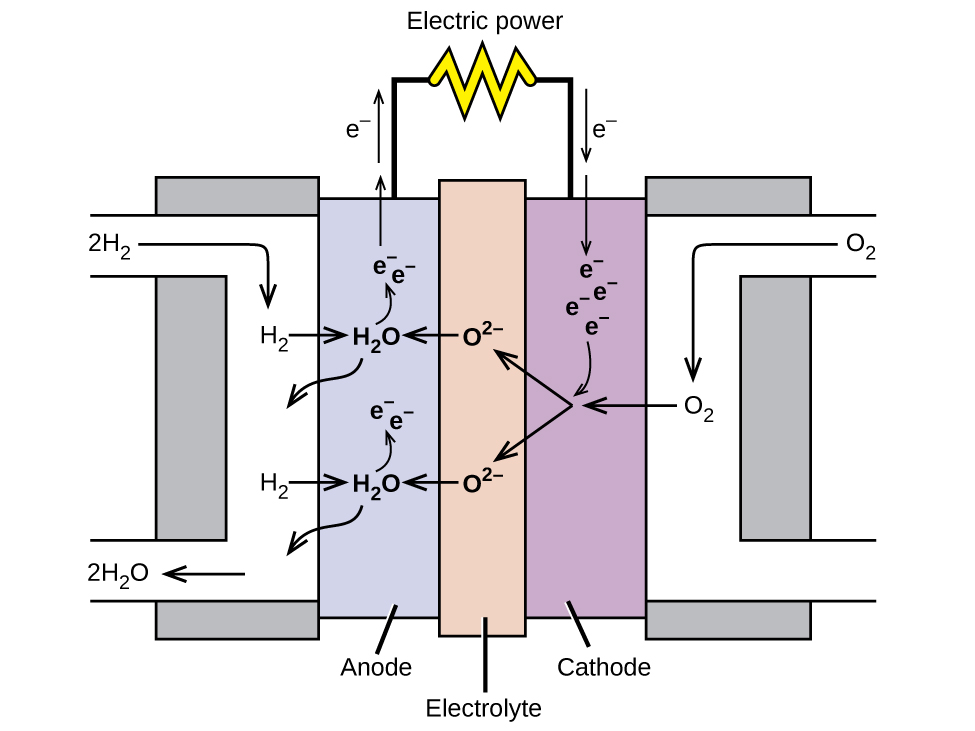
In a hydrogen fuel cell, the reactions are
| Anode: | 2 H2(g) + 2 O2-(aq) → 2 H2O(l) + 4 e– |
| Cathode: | O2(g) + 4 e– → 2 O2-(aq) |
| Overall: | 2 H2(aq) + O2(aq) → 2 H2O(l) |
The voltage produced about 0.9 V. The efficiency of fuel cells is typically about 40% to 60%, which is higher than the typical internal combustion engine (25% to 35%) and, in the case of the hydrogen fuel cell, produces only water as exhaust. Currently, fuel cells are rather expensive and contain features that cause them to fail after a relatively short time.

Check out this link to learn more about fuel cells.
Key Concepts and Summary
In this section, we look at a three applications of electrochemical cells. Primary batteries are single-use batteries often used in electronics that do not use large amounts of electricity (i.e. smoke alarms, remote controls, etc). Secondary batteries are rechargeable and are used when continuous electricity is needed (i.e. smartphones, portable computers, cars, etc). Fuel cells are an extremely efficient way to convert chemical energy into electrical energy and are used to supply power for satellites, space capsules, and some automobiles.
Glossary
alkaline battery
a common primary battery that used alkaline electrolytes, such as potassium hydroxide
battery
an electrochemical cell (or series of cells) that produce an electric current
fuel cell
a device that converts chemical energy into electrical energy and requires a continuous source of fuel (often hydrogen)
lead acid battery
a type of rechargeable battery used in your automobile to produce a high current required by automobile starter motors
lithium ion batteries
a popular rechargeable battery that consists of an anode reaction with LiCoO2 and a cathode reaction with Li and C6.
nickel-cadmium (NiCd)
a battery that consists of a nickel-plated cathode, cadmium-plated anode, and a potassium hydroxide electrode
primary battery
single-use batteries that cannot be recharged
secondary battery
batteries that can be recharged
Chemistry End of Section Exercises
- List some things that are typically considered when selecting a battery for a new application.
- Why do batteries go dead, but fuel cells do not?
- Explain why battery-powered electronics can perform poorly in low temperatures.
Answers to Chemistry End of Section Exercises
- Considerations include: cost of the materials used in the battery, toxicity of the various components (what constitutes proper disposal), should it be a primary or secondary battery, energy requirements (the “size” of the battery/how long should it last), will a particular battery leak when the new device is used according to directions, and its mass (the total mass of the new device).
- Batteries are self-contained and have a limited supply of reagents to expend before going dead. Alternatively, battery reaction byproducts accumulate and interfere with the reaction. Because a fuel cell is constantly resupplied with reactants and products are expelled, it can continue to function as long as reagents are supplied.
- Ecell, as described in the Nernst equation, has a term that is directly proportional to temperature. At low temperatures, this term is decreased, resulting in a lower cell voltage provided by the battery to the device—the same effect as a battery running dead.


Feedback/Errata