54 Electrolysis (M18Q8)
Learning Objectives
- Use reduction potentials to predict products of the electrolysis of molten salts and aqueous salts.
| Molten Salt | Aqueous Salt | - Calculate the quantity of product formed at an electrode during an electrolysis reaction or the amount of time required to generate a given amount of a species.
| Quantitative Electrolysis |
| Key Concepts and Summary | Key Equations | Glossary | End of Section Exercises |
Electrolysis
In galvanic cells, chemical energy is converted into electrical energy. The opposite is true for electrolytic cells. In electrolytic cells, electrical energy causes nonspontaneous redox reactions to occur in a process known as electrolysis.
The same principles are involved in electrolytic cells as in galvanic cells, with two differences:
- Electrons flow from the anion to the cation.
- Oxidation occurs at the anode.
- Reduction occurs at the cathode.
- The E°cell is negative and indicates a nonspontaneous process. Applying electrical energy to the system allows the nonspontaneous reaction to occur (Note, this is different from galvanic, or voltaic cells.)
- The anode is the POSITIVE electrode and the cathode is the NEGATIVE electrode (Note, this is different from galvanic, or voltaic cells.)
When determining the anode and cathode in an electrolytic cell:
- Identify all species in your system (usually ions and water (if an aqueous system))
- Determine which can be oxidized and which can be reduced to predict what happens at cathode and anode. Cations tend to not lose additional electrons and are reduced. Likewise, anions tend to not gain additional electrons and are oxidized. Water can be both oxidized and reduced, depending on the conditions.
Another way to analyze this is that the anode with a positive potential will attract negative ions, which will lose electrons as they are oxidized. The cathode with a negative potential will attract cations, which will gain electrons as they are reduced. A generic electrolytic cell can be seen in Figure 1.
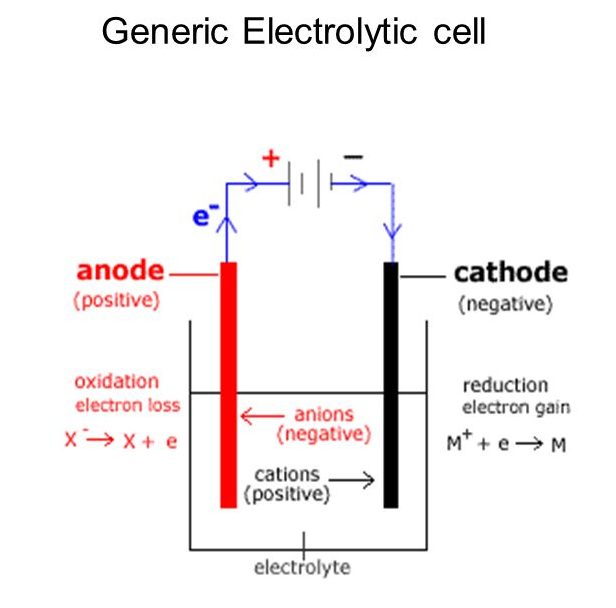
In this section, we will look at three electrolytic cells and the quantitative aspects of electrolysis.
The Electrolysis of Molten Sodium Chloride
Electrolysis of molten ionic compounds, or salts, can be used recreate the original elements in their non-ion form. A solid ionic compound has cations and anions locked in a crystal lattice structure that will not conduct electricity, however, melting the salt will allow the cations and anions of the salt to move freely and conduct electricity. This means it is possible to apply an electrical current to the molten salt and drive the nonspontaneous redox reactions to form the original elements in their standard state.
In molten sodium chloride, liquid sodium and chloride ions are present. Sodium ions already have a positive charge and are reduced. Likewise, chloride ions already have a negative charge and are oxidized. In the electrolytic cell, the ions are free to migrate to the electrodes. A simplified diagram of the cell commercially used to produce sodium metal and chlorine gas is shown in Figure 2. Sodium is a strong reducing agent and chlorine is used to purify water, and is used in antiseptics and in paper production. The reactions are:
| Anode: | 2 Cl-(l) → Cl2(g) + 2 e- | E° = +1.3 V |
| Cathode: | Na+(l) + e- → Na(l) | E° = -2.7 V |
| Overall: | 2 Na+(l) + 2 Cl-(l) → 2 Na(l) + Cl2(g) | E°cell = -4.0 V |
The power supply (battery) must supply a minimum of 4 V.
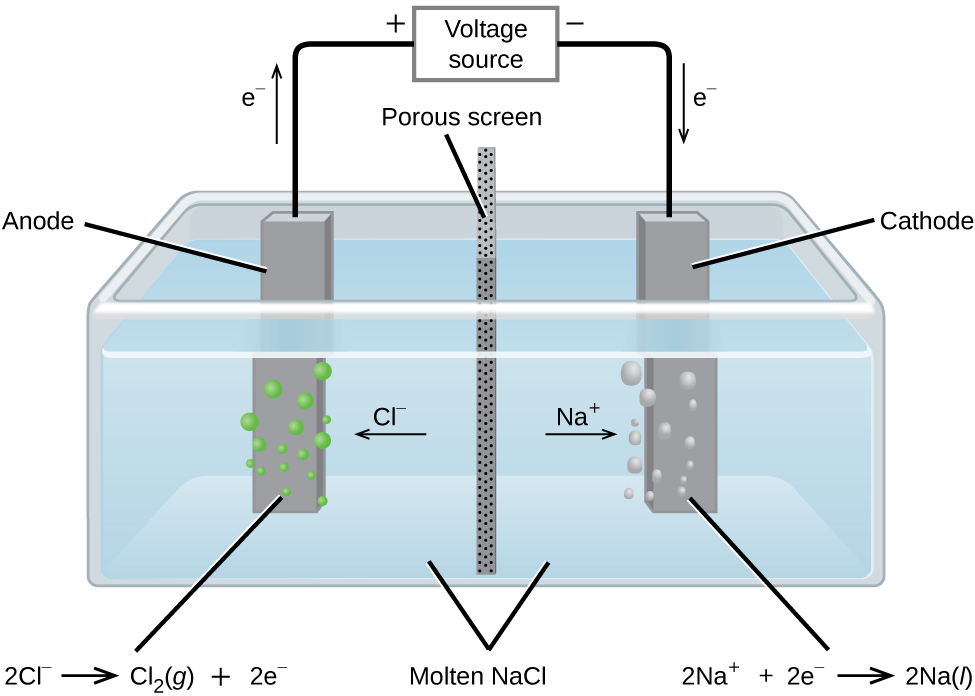
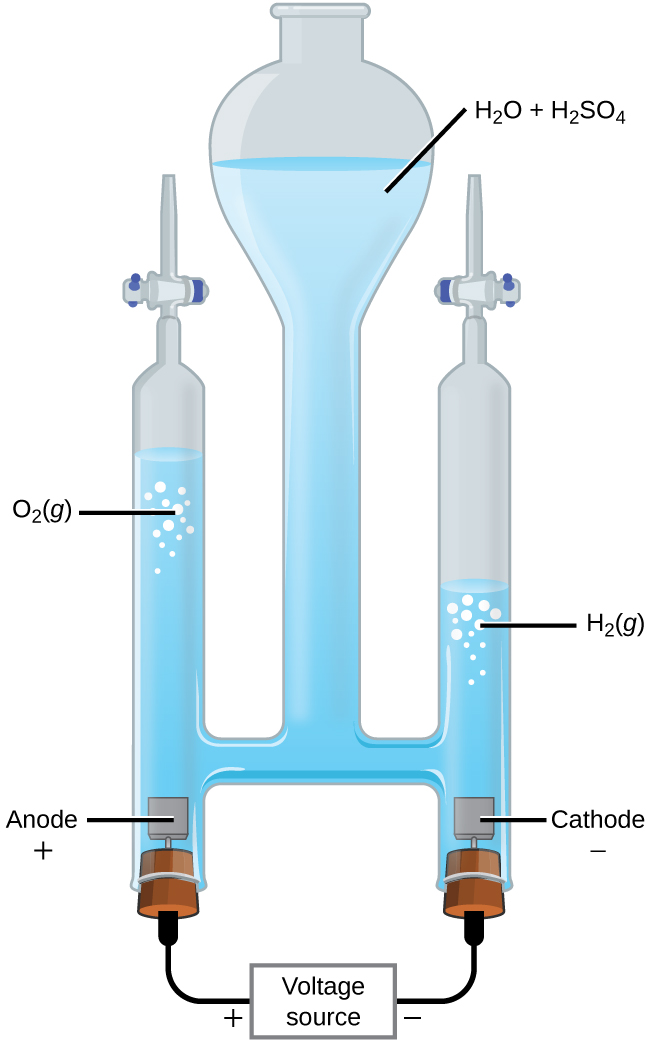
The Electrolysis of Water
It is possible to split water into hydrogen and oxygen gas by electrolysis. Acids are typically added to increase the concentration of hydrogen ion in solution (Figure 3). The reactions are:
| Anode: | 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e- | E° = +1.23 V |
| Cathode: | 2 H+(aq) + 2 e- → H2(g) | E° = 0 V |
| Overall: | 2 H2O(l) → O2(g) + 2 H2(g) | E°cell = -1.23 V |
The overall voltage is -1.23 V, which indicate a non-spontaneous reaction. A minimum voltage of 1.23 V must be applied to the reaction system in order to cause the electrolysis of water to happen.
The Electrolysis of Aqueous Sodium Iodide
The electrolysis of aqueous solutions are an interesting example of electrolysis. When the ionic compound dissolves into cations and anions, a solution capable of conducting electricity is produced. The cations, anions and water (solvent) will create multiple possible oxidation and reductions reaction. Ultimately, the oxidation and reduction reactions that occur will be the ones that create the smallest Ecell value .
- At the anode, either the anion of the salt or the water will be oxidized, whichever is the stronger reducing agent.
- At the cathode either the cation or the water will be reduced, whichever is the strongest oxidizing agent.
Consider the electrolysis of 1 M aqueous sodium iodide, the sodium iodide dissolves in the water so sodium ions, iodide ions, and water are all present in the solution.
Looking at the anode first, either the iodide ion or the water will be oxidized. The possible reactions are (note: E°red values given from the standard reduction potential table):
| (i) 2 I-(aq) → I2(g) + 2 e- | E° = +0.54 V |
| (ii) 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e- | E° = +1.23 V |
For electrolysis to occur, voltage must be applied to overcome the negative Ecell. When multiple reactions are possible, the reactions that result in the least negative Ecell will be the ones that occur. This means that the oxidation reaction with the least positive E° will happen. The values above suggest that iodide ions will be oxidized at the anode as using reaction (1) for the oxidation would give a less-negative cell potential.
Now consider the cathode. Two possible reductions could occur:
| (iii) 2 H2O(l) + 2 e- → H2(g) + 2 OH-(aq) | E° = -0.83 V |
| (iv) Na+(aq) + e- → Na(s) | E° = -2.71 V |
The reduction reaction with the least negative (or most positive) will occur. This means when voltage is applied to the reaction system, reaction (iii) will occur.
The overall reaction is then:
| Anode: | 2 I-(aq) → I2(g) + 2 e- | E° = +0.54 V |
| Cathode: | 2 H2O(l) + 2 e- → H2(g) + 2 OH-(aq) | E° = -0.83 V |
| Overall: | 2 H2O(l) + 2 I-(aq) → H2(g) + 2 OH-(aq) + I2(g) | E°cell = -1.37 V |
Chemistry in Real Life: Electroplating
An important use for electrolytic cells is in electroplating. Electroplating results in a thin coating of one metal on top of a conducting surface. Reasons for electroplating include making the object more corrosion resistant, strengthening the surface, producing a more attractive finish, or for purifying metal. The metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. Common consumer products include silver-plated or gold-plated tableware, chrome-plated automobile parts, and jewelry. We can get an idea of how this works by investigating how silver-plated tableware is produced (Figure 4).
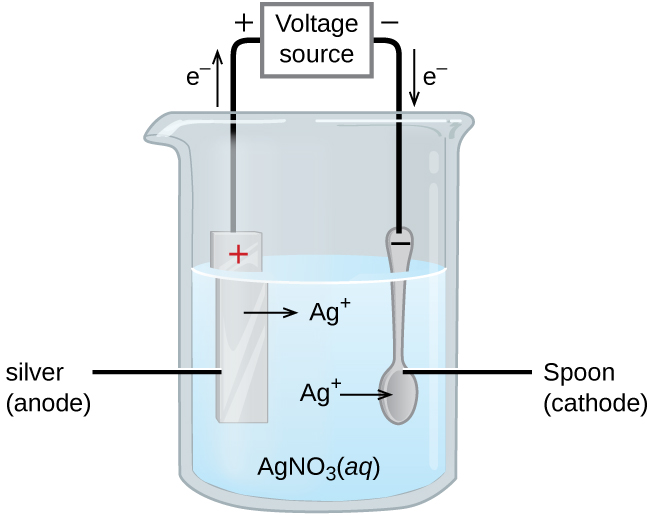
In the figure, the anode consists of a silver electrode, shown on the left. The cathode is located on the right and is the spoon, which is made from inexpensive metal. Both electrodes are immersed in a solution of silver nitrate. As the potential is increased, current flows. Silver metal is lost at the anode as it goes into solution:
Ag(s) → Ag+(aq) + e-
The mass of the cathode increases as silver ions from the solution are deposited onto the spoon:
Ag+(aq) + e- → Ag(s)
The net result is the transfer of silver metal from the anode to the cathode. The quality of the object is usually determined by the thickness of the deposited silver and the rate of deposition.
Quantitative Aspects of Electrolysis
The current applied over a given amount of time can be used to quantitatively find the amount of material electroplated. The amount of material electroplated also depends on the number of moles of electrons transferred during the reaction, using the equation:
Amps × time = [latex]\frac{(mass\ plated)(mol\ e^{-}\ transferred)(F)}{Molar\ Mass}[/latex]
Where Amps = Coulombs/sec, time = seconds, mass plated = grams, F = 96485 C/mol e-, and Molar Mass = g/mol of molecule electroplated (usually a metal).
Example 1
Converting Current to Mass of Solid Electroplated
In one process used for electroplating silver, a current of 10.23 A was passed through an electrolytic cell for exactly 1 hour. What mass of silver was deposited at the cathode from a silver nitrate solution?
Solution
We can use the equation from above in order to calculate the mass of silver electroplated. First, to find the number of electrons transferred in the process, we need to look at AgNO3(aq) being converted in Ag(s). The balanced half-reaction for this process would be: Ag+(aq) + e- → Ag(s). One mole of electrons is transferred in the process.
We also need to convert the time into seconds. One hour is 3600 seconds.
Lastly, the molar mass of silver is 107.86 g/mol.
Amps × time = [latex]\frac{(mass\ plated)(mol\ e^{-}\ transferred)(F)}{Molar\ Mass}[/latex]
(10.23 Amps) (3600 sec) = [latex]\frac{(mass\ plated)(1\ mol\ e^{-})(96485\ C/mol\ e-)}{107.86\ g/mol}[/latex]
Solving for the mass of silver plated, we find that 41.19 g of Ag is electroplated.
Check Your Learning
Aluminum metal can be made from aluminum ions by electrolysis. What is the half-reaction at the cathode? What mass of aluminum metal would be recovered if a current of 2.50 × 103 A passed through the solution for 15.0 minutes?
Answer:
Al3+(aq) + 3 e- → Al(s); 210. g Al
Example 2
Time Required for Deposition
In one application, a 0.010-mm layer of chromium must be deposited on a part with a total surface area of 3.3 m2 from a solution containing chromium(III) ions. How long would it take to deposit the layer of chromium if the current is 33.46 A? The density of chromium (metal) is 7.19 g/cm3.
Solution
This problem brings in a number of topics covered earlier. First, we must find the amount of chromium deposited using the density and the volume of Cr required:
Volume chromium needed = length × width × height = surface area × height
Volume chromium needed = (3.3 m2) × (0.010 mm × [latex]\frac{1\ m}{1000\ mm}[/latex] = 3.3 × 10-5 m3
Mass chromium deposited = density × volume
Mass chromium deposited = (7.19 g/cm3) × (3.3 × 10-5 m3) × ([latex]\frac{100\ cm}{1\ m}[/latex])3 = 237.3 g chromium
Additionally, since the solution contains chromium(III) ions (Cr3+), 3 moles of electrons are required per mole of Cr. Now, we can calculate the amount of time required:
Amps × time = [latex]\frac{(mass\ plated)(mol\ e^{-}\ transferred)(F)}{Molar\ Mass}[/latex]
(33.46 Amps) (time) = [latex]\frac{(237.3\ grams)(3\ mol\ e^{-})(96485\ C/mol\ e-)}{51.996\ g/mol}[/latex]
time = 39000 seconds, or 11 hours
Key Concepts and Summary
Electrolytic cells are often thought of as the opposite of galvanic cells, where nonspontaneous reactions occur through an application of electrical energy. Many of the same principles that apply to a galvanic cell also apply to an electrolytic cell, except for the "charges" given to the anode and cathode.
When considering the electrolysis of a molten salt, the anion is always oxidized at the anode and the cation is always reduced at the cathode. In order to make a reaction occur, a voltage greater than the electrochemical cell potential (Eºcell) must be applied. During electrolysis of an aqueous salt, there are two possible anodes (the anion and water) and two possible cathodes (the cation and water). In order to know whether the anion or water will be oxidized, we look at the standard reduction potential table and choose the species that is the stronger reducing agent, or has the least positive standard reduction potential. In order to know whether the cation or water will be reduced, we choose the species that is the stronger oxidizing agent, or the species that has the least negative standard reduction potential. By doing this, we minimize the voltage that must be applied in order to allow the nonspontaneous reaction to occur.
We can also calculate many aspects of electrolysis quantitatively, including the current that must be applied (in Amps) the time required, and the mass of metal plated.
Key Equations
- Amps × time = [latex]\frac{(mass\ plated)(mol\ e^{-}\ transferred)(F)}{Molar\ Mass}[/latex]
Glossary
electrolysis
a process where electrical energy causes nonspontaneous redox reactions to occur
electrolytic cell
an electrochemical cell which involves a nonspontaneous reaction, as opposed to the spontaneous reactions in a galvanic cell
electroplating
a process that results in a thin coating of one metal on top of a conducting surface, often another metal
Chemistry End of Section Exercises
- Consider the electroylsis of molten CaI2, what reactions will occur at the cathode and the anode? How many grams of calcium metal will be produced by supplying the system with 0.75 amps for 50 minutes?
- An attempt is made to produce Na metal by electrolyzing an aqueous Na2SO4 solution with a pH close to 7. Will it be successful? Explain.
- Considering only the cost of electricity, would it be most expensive to produce 100.0 grams of solid aluminum (from Al(NO3)3), gold (from AuNO3), or zinc (from Zn(NO3)2?
- Hydrogen for fuel cells can be produced via electrolysis.
- What is the potential required for this reaction?
- With a cost of 15 cents per kilowatt-hour, how much will it cost to produce 1.00 kilograms of hydrogen gas?
Answers to Chemistry End of Section Exercises
- 0.47 g; anode: 2 I-(l) → I2(s) + 2 e-; cathode: Ca2+(l) + 2 e- → Ca(s)
- No. Reduction of sodium at the cathode (Na+(aq) + e- → Na(s); E° = -2.71 V) competes with the reduction of H+ (2 H+(aq) + 2 e- → H2(g); E° = 0.00 V) and the reduction of H2O (2 H2O(l) + 2 e- → H2(g) + OH-(aq); E° = -0.83 V). The sodium reaction has the most negative E° and so will not occur.
- Aluminum has the most negative E° so it will require the most electricity to reduce.
-
- E° = -1.23 V; The minimum potential required is 1.23 V.
- $4.92


Feedback/Errata