6 Functional groups: Suffixes/prefixes, Isomers, Intermolecular Forces (M12Q5)
Learning Objectives
- Identify functional groups in molecular structures and draw organic structures containing selected functional groups.
| Alcohol | Ether | Aldehyde | Ketone | Carboxylic Acid | Ester | Amine | Amide | - Using suffix information, associate structures containing a single functional group to their names.
| Summary Table | Intermolecular Forces | - Categorize primary, secondary, and tertiary alcohols and amines.
| Alcohols | Amines |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Alcohols and Ethers
Incorporation of just one oxygen atom into an organic molecule can take the form of several different functional groups. When the oxygen atom is attached by single bonds, the molecule is either an alcohol or ether. Alcohols and ethers can be structural isomers of each other.
Alcohols
Alcohols are derivatives of hydrocarbons in which an –OH group has replaced a hydrogen atom. Although all alcohols have one or more hydroxyl (–OH) functional groups, they do not behave like bases such as NaOH and KOH. NaOH and KOH are ionic compounds that contain OH– ions. Alcohols are covalent molecules; the –OH group in an alcohol molecule is attached to a carbon atom by a covalent bond.
The name of an alcohol comes from the hydrocarbon from which it was derived. The final -e in the name of the hydrocarbon is replaced by -ol, and the carbon atom to which the –OH group is bonded is indicated by a number placed before the name.[1]
Example 1
Naming Alcohols
Consider the following example. How should it be named?
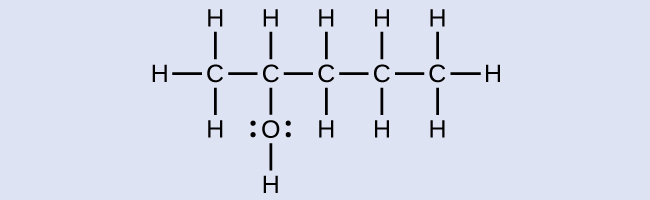
Solution
The carbon chain contains five carbon atoms. If the hydroxyl group was not present, we would have named this molecule pentane. To address the fact that the hydroxyl group is present, we change the ending of the name to -ol. In this case, since the –OH is attached to carbon 2 in the chain, we would name this molecule 2-pentanol.
Check Your Learning
Name the following molecule:
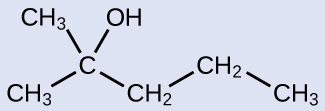
Answer:
2-methyl-2-pentanol
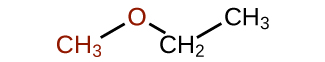
Ethers
Ethers are compounds that contain the functional group C–O–C, where both C atoms are sp3 hybridized. In the general formula for ethers, R—O—R, the hydrocarbon groups (R) may be the same or different. Ethers do not have a designated suffix like the other types of molecules we have named so far. For naming ethers, the two branches connected to the oxygen atom are named separately (and alphabetically), followed by “ether.” The name for the compound shown below is ethylmethyl ether:
Example 2
Naming Ethers
Provide the name for the ether shown here:
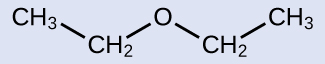
Solution
The groups attached to the oxygen atom are both ethyl groups, so the common name would be diethyl ether.
Check Your Learning
Provide the name for the ether shown:
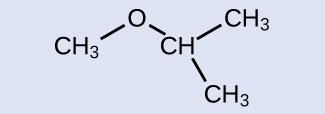
Answer:
isopropylmethyl ether
Both aldehydes and ketones contain a carbonyl group, a functional group with a carbon-oxygen double bond. The names for aldehyde and ketone compounds are derived using similar nomenclature rules as for alkanes and alcohols, and include the class-identifying suffixes -al and -one, respectively:
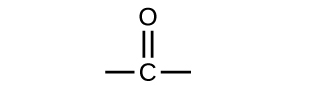
In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. In a ketone, the carbonyl group is bonded to two carbon atoms:
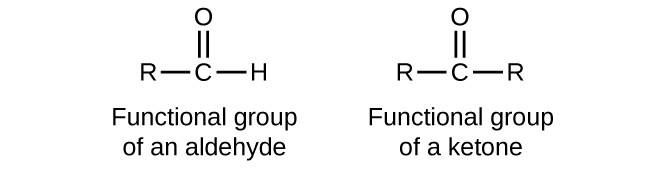
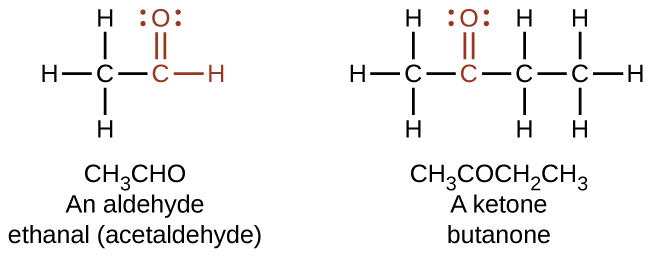
In a condensed formula, an aldehyde group is represented as –CHO; a ketone is represented as –C(O)– or –CO–. Aldehydes and ketones can be structural isomers of each other.
In both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar; the carbon atom exhibits sp2 hybridization. Two of the sp2 orbitals on the carbon atom in the carbonyl group are used to form σ bonds to the other carbon or hydrogen atoms in a molecule. The remaining sp2 hybrid orbital forms a σ bond to the oxygen atom. The unhybridized p orbital on the carbon atom in the carbonyl group overlaps a p orbital on the oxygen atom to form the π bond in the double bond.
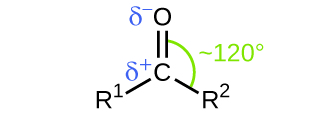
Like the C=O bond in carbon dioxide, the C=O bond of a carbonyl group is polar (recall that oxygen is significantly more electronegative than carbon, and the shared electrons are pulled toward the oxygen atom and away from the carbon atom) (Figure 1).
Carboxylic Acids and Esters
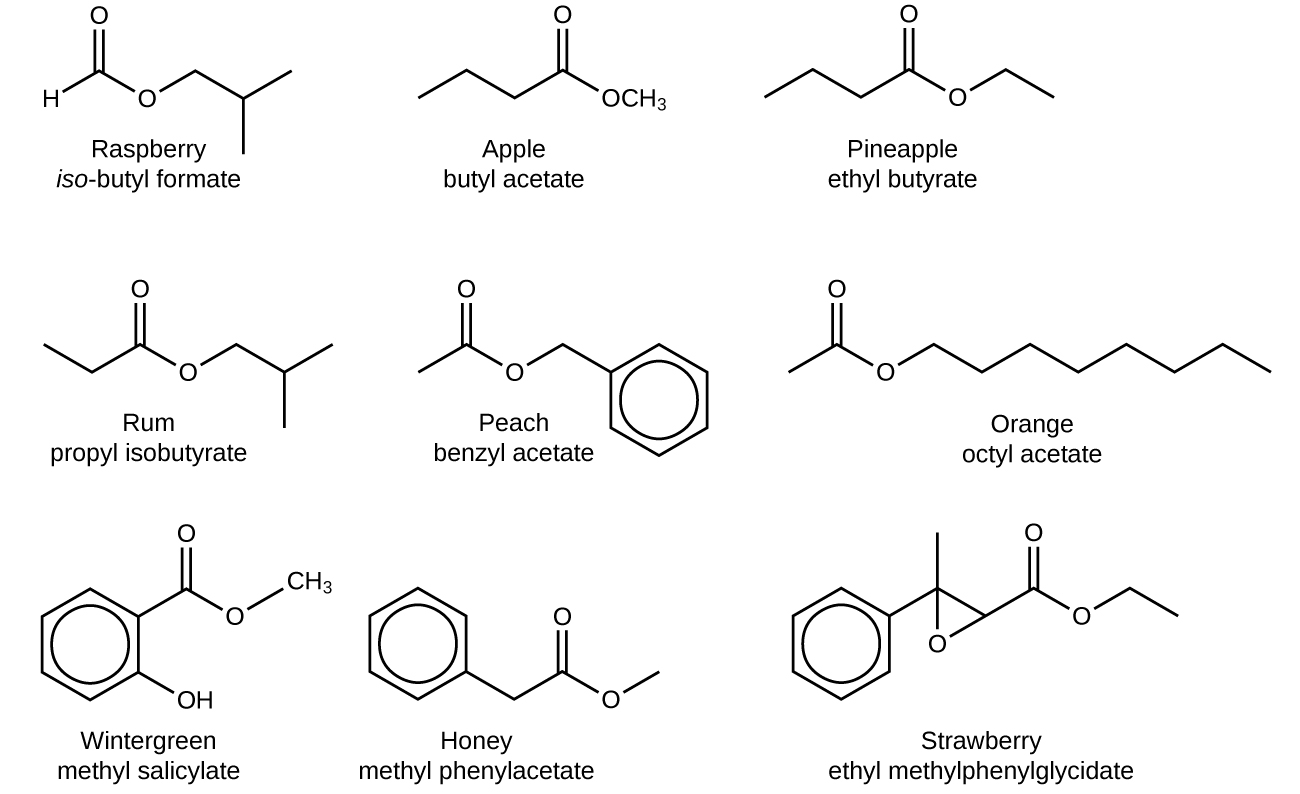
The odor of vinegar is caused by the presence of acetic acid, a carboxylic acid, in the vinegar. The odor of ripe bananas and many other fruits is due to the presence of esters, compounds that can be prepared by the reaction of a carboxylic acid with an alcohol (covered in the next chapter). Some common esters and their odors are in Figure 2, for your reference.
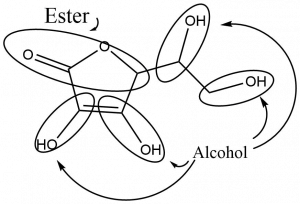
Both carboxylic acids and esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond. In a carboxylic acid, the second oxygen atom also bonds to a hydrogen atom. In an ester, the second oxygen atom bonds to another carbon atom. The names for carboxylic acids and esters include prefixes that denote the lengths of the carbon chains in the molecules and suffixes that denote the type of functional group. The suffixes are “-oic acid” is a carboxylic acid containing molecule, and that “-oate” is an ester containing molecule.
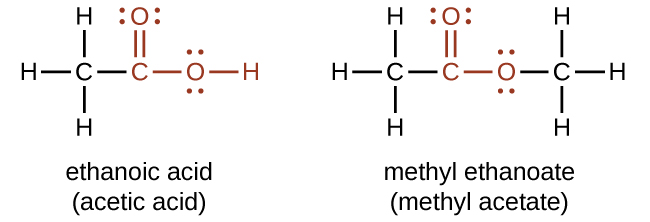
The functional groups for an acid and for an ester are shown in red in these formulas.
Carboxylic acids are weak acids (see the chapter on acids and bases), meaning they are not 100% ionized in water. Generally only about 1% of the molecules of a carboxylic acid dissolved in water are ionized at any given time. The remaining molecules are undissociated in solution.
Chemistry in Real Life: Carboxylic Acids
The simplest carboxylic acid is formic acid, HCO2H, known since 1670. Its name comes from the Latin word formicus, which means “ant”; it was first isolated by the distillation of red ants. It is partially responsible for the pain and irritation of ant and wasp stings, and is responsible for a characteristic odor of ants that can be sometimes detected in their nests.
Acetic acid, CH3CO2H, constitutes 3–6% vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present. Yeast cells present in the juice carry out the fermentation reactions. The fermentation reactions change the sugar present in the juice to ethanol, then to acetic acid. Pure acetic acid has a penetrating odor and produces painful burns. It is an excellent solvent for many organic and some inorganic compounds, and it is essential in the production of cellulose acetate, a component of many synthetic fibers such as rayon.
The distinctive and attractive odors and flavors of many flowers, perfumes, and ripe fruits are due to the presence of one or more esters (Figure 3). Among the most important of the natural esters are fats (such as lard, tallow, and butter) and oils (such as linseed, cottonseed, and olive oils), which are esters of the trihydroxyl alcohol glycerine, C3H5(OH)3, with large carboxylic acids, such as palmitic acid, CH3(CH2)14CO2H, stearic acid, CH3(CH2)16CO2H, and oleic acid, CH3(CH2)7CH=CH(CH2)7CO2H. Oleic acid is an unsaturated acid; it contains a C=C double bond. Palmitic and stearic acids are saturated acids that contain no double or triple bonds.

Amines
Amines are molecules that contain carbon-nitrogen bonds. The nitrogen atom in an amine has a lone pair of electrons and three bonds to other atoms, either carbon or hydrogen. Naming amines can be somewhat complicated, but the name should always include the class-identifying suffix amine as in the following examples:
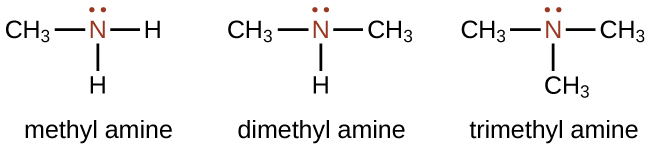
In the structures above, the first structure, methyl amine, is a primary amine since the nitrogen is attached to one carbon atom. The second structure, dimethyl amine, is a secondary amine since the nitrogen is attached to two carbon atoms. The third structure, trimethyl amine, is a tertiary amine since the nitrogen is attached to three carbon atoms. The reactivity of primary and secondary amines is different than that of tertiary amines, which will be covered in the next chapter.
Chemistry in Real Life: Addictive Alkaloids
Since ancient times, plants have been used for medicinal purposes. One class of substances, called alkaloids, found in many of these plants has been isolated and found to contain cyclic molecules with an amine functional group. These amines are bases. They can react with H3O+ in a dilute acid to form an ammonium salt, and this property is used to extract them from the plant:
The name alkaloid means “like an alkali.” Thus, an alkaloid reacts with acid. The free compound can be recovered after extraction by reaction with a base:
The structures of many naturally occurring alkaloids have profound physiological and psychotropic effects in humans. Examples of these drugs include nicotine, morphine, codeine, and heroin. The plant produces these substances, collectively called secondary plant compounds, as chemical defenses against the numerous pests that attempt to feed on the plant:
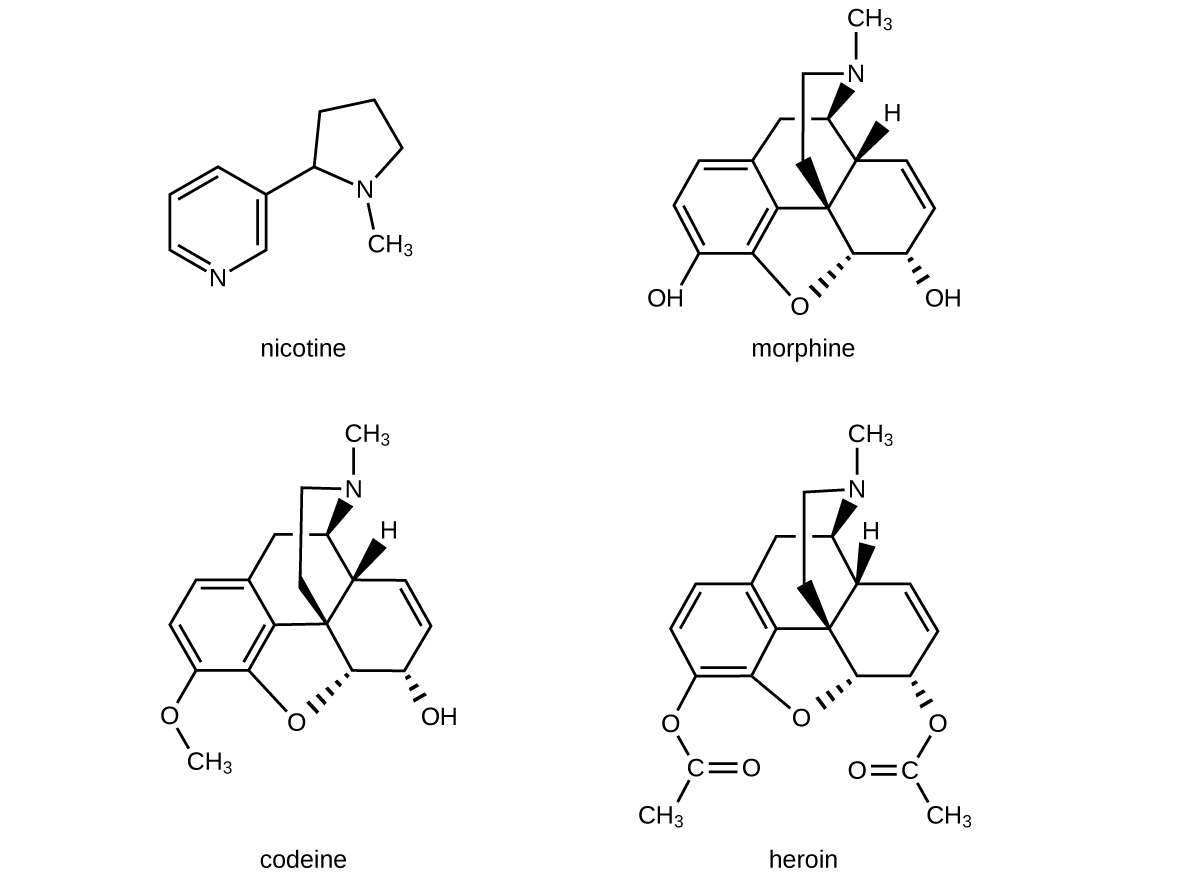
In these diagrams, as is common in representing structures of large organic compounds, carbon atoms in the rings and the hydrogen atoms bonded to them have been omitted for clarity. The solid wedges indicate bonds that extend out of the page. The dashed wedges indicate bonds that extend into the page. Notice that small changes to a part of the molecule change the properties of morphine, codeine, and heroin. Morphine, a strong narcotic used to relieve pain, contains two hydroxyl functional groups, located at the bottom of the molecule in this structural formula. Changing one of these hydroxyl groups to a methyl ether group forms codeine, a less potent drug used as a local anesthetic. If both hydroxyl groups are converted to esters of acetic acid, the powerfully addictive drug heroin results (Figure 6).

Amides (or Carboxamides)
Amides are molecules that contain nitrogen atoms connected to the carbon atom of a carbonyl group. The naming of amides is complex and beyond the scope of this course, but all names include the use of the suffix -amide:
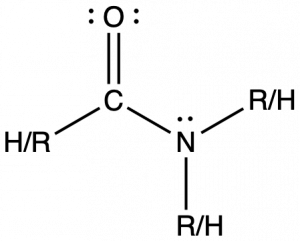
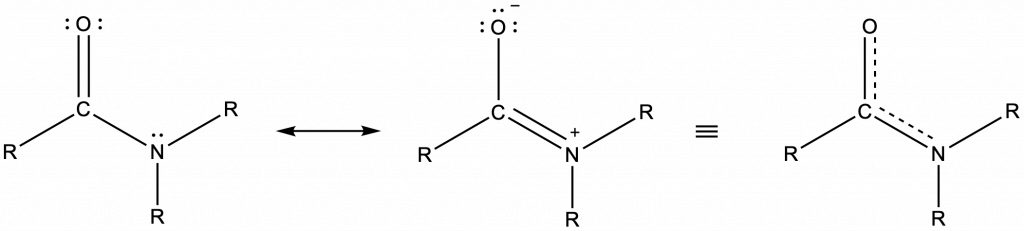
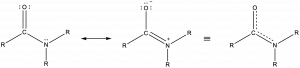
Amide functional groups exhibit resonance, which affects their physical structure and reactivity. Below, the left resonance structure is drawn with C=O and C-N (as above) but the other resonance structure is drawn with C-O and C=N bonds (center structure). The net result is that the π-bond electrons delocalize between the O-C-N electron regions and give the O, C, and N sp2 hybridization and 120º bond angles (right structure). Even though amides are typically drawn with O=C-N bonding (left structure), it is important to remember the effects of resonance on the geometry of the molecule.
It is worth noting that some functional groups, like amides, appear to include smaller functional groups, like an amine, so you might be tempted to describe an amide as two functional groups: a carbonyl and an amine. The same idea can be used to think of a carboxylic acid as a ketone and an alcohol. However, this is not the correct approach. In such cases, the largest set of connected atoms that make a up a known functional group should be identified as one functional group, and not multiple. The table here summarizes the structures discussed in this section: 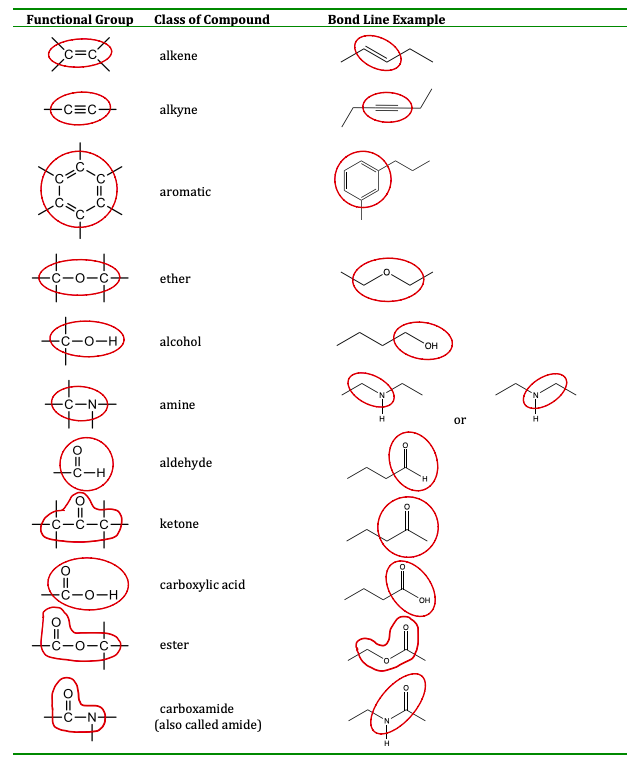
Intermolecular Forces
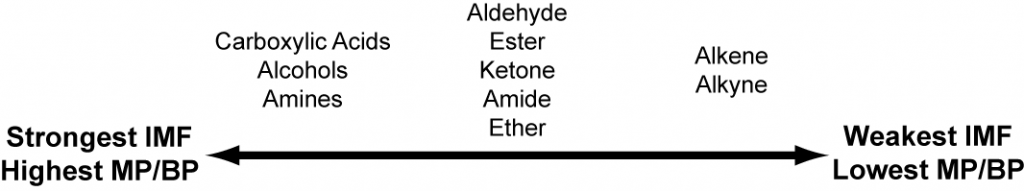
The functional groups within a molecule determine its physical properties, like melting and boiling points. Certain functional groups, like carboxylic acids and alcohols, have hydrogen-bonding abilities. Other functional groups, like ethers and ketones, are polar. Hydrocarbon functional groups, like alkenes and alkynes, are only able to have LDF.
In general, assuming carbon chains of equal length:
Key Concepts and Summary
This section is extremely important in your understanding of organic chemistry. The physical property of a molecule and the way it reacts with another molecule is based on the functional groups it contains. In previous sections, we looked at functional groups that were only between carbon atoms (alkenes, alkynes), but in this section, we expand to molecules that also contain oxygen and nitrogen. The presence of oxygen and nitrogen containing functional groups is important as many of these molecules contain a molecular dipole, have stronger intermolecular forces, and have higher melting/boiling points or lower vapor pressures that similar molecules without those functional groups. A brief description of each functional group is given in the glossary below, while its structure is circled in the figure above.
Glossary
alcohol (-ol)
a molecule where an -OH group has replaced a hydrogen
aldehyde (-al)
a molecule with a terminal carbon single bonded to a hydrogen and double bonded to an oxygen
amide (carboxamide, –amide)
a molecule that contains a central carbon double bonded to an oxygen and single bonded to a nitrogen.
amine (- amine)
a molecule that contains a nitrogen with a lone pair and single bonded to three different groups
carbonyl
a carbon-oxygen double bond
carboxylic acid (-oic acid)
a molecule with a terminal carbon single bonded to an -OH group and double bonded to an oxygen
ester (-oate)
a molecule with a central carbon double bonded to an oxygen and single bonded to an oxygen.
ether (- ether)
a molecule that contains C-O-C, where both carbons are sp3 hybridized.
functional group
a specific set of atoms that has characteristic properties
ketone (-one)
a molecule with a central carbon double bonded to an oxygen. This carbon is never a terminal carbon and must be single bonded to two other carbons, often written as C-C(O)-C.
Chemistry End of Section Exercises
- Several oxygen-containing functional groups are covered in this chapter. How might you recognize them from the name of a molecule?
- Which of the following molecules would you expect to have the highest boiling point: 1-butanol, butanal, or 2-butanone? Explain.
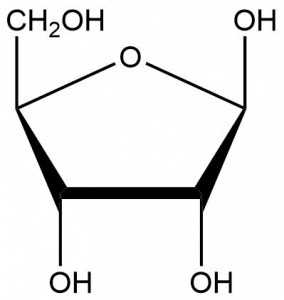
- D-ribose, often depicted as shown below, is a simple sugar. It is a component of the ribonucleotides that make up RNA. What functional groups are present? The chirality of this molecule is biologically important. How many chiral carbons can be found in this molecule?
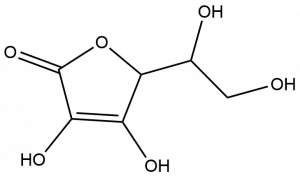
- Ascorbic acid, or vitamin C, plays an important role in the body. It is needed to maintain the health of skin, cartilage, teeth, bone, and blood vessels and to protect your body’s cells from damage. From the structure shown below, determine and circle the oxygen-containing functional groups present in vitamin C.
- All of the compounds below have a similar molecular weight, and therefore comparable London dispersion forces. Rank these compounds in order of lowest to highest boiling point. Explain your strategy for answering this question.
- 3-hexanone
- hexanoic acid
- 3-methylhexane
- 3-hexanol
- Looking at the molecule names in question 5, why is there no number prefix present in hexanoic acid?
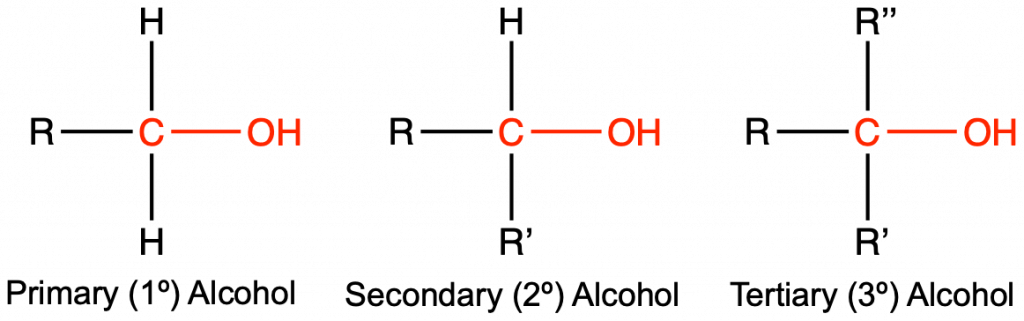
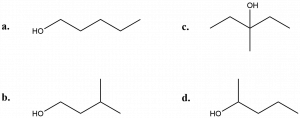
- Determine whether each of the following alcohols is a primary (1°), secondary (2°) or tertiary alcohol (3°):
- The lone pair of electrons on the nitrogen in amine compounds make these compounds basic. Amides, however, are neutral, not basic. How might this be explained?
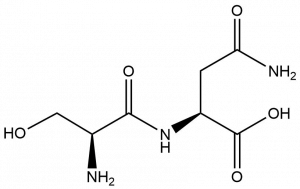
- What functional groups do you find in the following dinucleotide?
Answers to Chemistry End of Section Exercises
- The suffix of the name can indicate what type of function group is present. The following table summarizes this information:
Functional Group Suffix Alcohol -ol Ether ether (Note: Adding the word ether is a colloquial way to name ethers. IUPAC naming for ethers is more complicated and beyond this course) Aldehyde -al Ketone -one Carboxylic Acid -oic acid Ester -oate - 1-butanol. The intermolecular forces found in 1-butanol (an alcohol) are the strongest, leading to the highest boiling point.
- D-ribose has four alcohol groups and one ether group. Four out of the five carbons present are chiral.
- This question is asking about boiling points, which is determined by the molecule’s strength of intermolecular forces.
All of the molecules are affected by London dispersion forces, however, the problem indicates that all of the molecules have similar molar masses so one can assume the London dispersion forces all equivalent in all of the molecules and will not be a determining factor for boiling points.
After using the suffix name to find the functional group present in each molecule, the intermolecular forces for each molecule can be determined:- 3-hexanone: The -one indicates this molecule is a ketone. Ketone functional groups produce dipole-dipole intermolecular forces.
- Hexanoic acid: The -oic acid indicates this molecule is a carboxylic acid. Carboxylic acid functional groups produce hydrogen bonding intermolecular forces. Note that there are two oxygen atoms where hydrogen bonding can take place.
- 3-methylhexane: The -ane indicates this is an alkane molecule. Alkanes are only affected by London Dispersion forces.
- 3-Hexanol: The -ol ending indicate this molecule is an alcohol. Alcohol functional groups produce hydrogen bonding intermolecular forces. Note that there is only one oxygen atom where hydrogen bonding can take place.
Based on intermolecular force strength, boiling point order is as follows (actual boiling points are given for verification):
3-methylhexane (92°C) < 3-hexanone (123°C) < 3-hexanol (135°C) < hexanoic acid (206°C) - Carboxylic acids are terminal functional groups – they will always be on an end of the carbon chain.
- (a) 1°; (b) 1°; (c) 3°; (d) 2°
- The electron pair in the amide is not free to behave as a base but is involved in a delocalized pi bond with the carbonyl group.
- Alcohol (1), amine (1), amide (2), carboxylic acid (1)


Feedback/Errata