32 Lewis Acids and Bases (M15Q8)
Learning Objectives
- Identify Lewis acids and bases.
| Key Concepts and Summary | Glossary | End of Section Exercises |
In 1923, G. N. Lewis proposed a generalized definition of acid-base behavior in which acids and bases are identified by their ability to accept or to donate a pair of electrons and form a coordinate covalent bond.
A coordinate covalent bond (or dative bond) occurs when one of the atoms in the bond provides both bonding electrons. For example, a coordinate covalent bond occurs when a water molecule combines with a hydrogen ion to form a hydronium ion. A coordinate covalent bond also results when an ammonia molecule combines with a hydrogen ion to form an ammonium ion. Both of these equations are shown here.
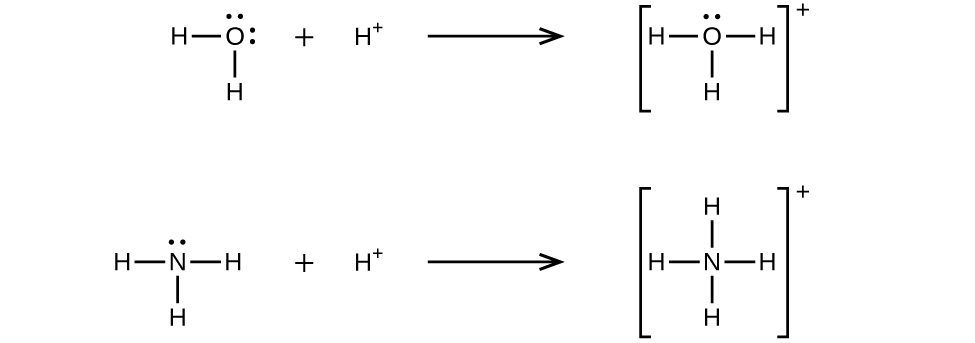
A Lewis acid is any species (molecule or ion) that can accept a pair of electrons, and a Lewis base is any species (molecule or ion) that can donate a pair of electrons.
A Lewis acid-base reaction occurs when a base donates a pair of electrons to an acid. The product contains a newly-formed coordinate covalent bond between the Lewis acid and the Lewis base. The following equations illustrate the general application of the Lewis concept.
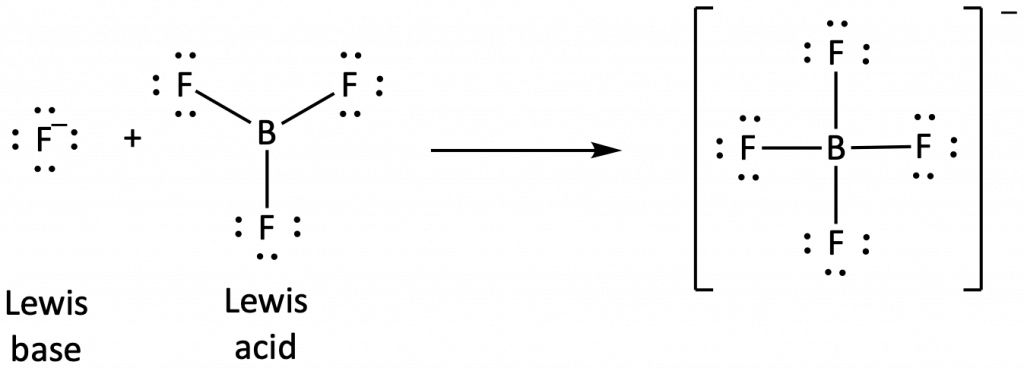
The boron atom in boron trifluoride, BF3, has only six electrons in its valence shell. Being short of the preferred octet, BF3 is a very good Lewis acid and reacts with many Lewis bases; a fluoride ion is the Lewis base in this reaction, donating one of its lone pairs:
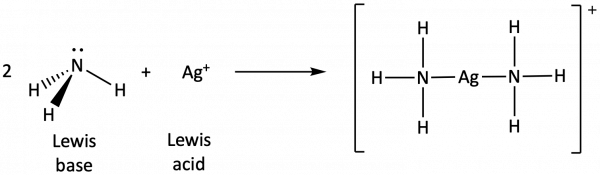
In the following reaction, each of two ammonia molecules, Lewis bases, donates a pair of electrons to a silver ion, the Lewis acid:
Key Concepts and Summary
The final definition of acids and bases we will learn in this course is the most comprehensive as they only require molecules to transfer a pair of electrons, rather than a proton. Lewis acids are defined as accepting a pair of electrons and Lewis bases as donating a pair of electrons. A Lewis acid tends to be more positively charged and a Lewis base more negatively charged (although this is not robust enough for all scenarios!)
Glossary
coordinate covalent bond
a covalent bond where one of the atoms in the bond provides both bonding electrons, rather than each atom providing a single electron to form the bond
Lewis acid
a species (molecule or ion) that can accept a pair of electrons
Lewis base
a species (molecule or ion) that can donate a pair of electrons
Chemistry End of Section Exercises
- Write the Lewis structures of the reactants and product of each of the following equations, and identify the Lewis acid and the Lewis base in each:
- CO2(g) + OH–(aq) ⇌ HCO3–(aq)
- B(OH)3(aq) + OH–(aq) ⇌ B(OH)4–(aq)
- I–(aq) + I2(aq) ⇌ I3–(aq)
- AlCl3(s) + Cl–(aq) ⇌ AlCl4–(aq)
- O2-(aq) + SO3(aq) ⇌ SO42-(aq)
Answers to Chemistry End of Section Exercises
- (a)
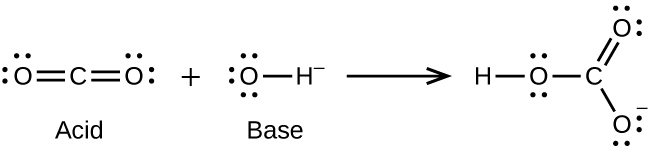
(b)
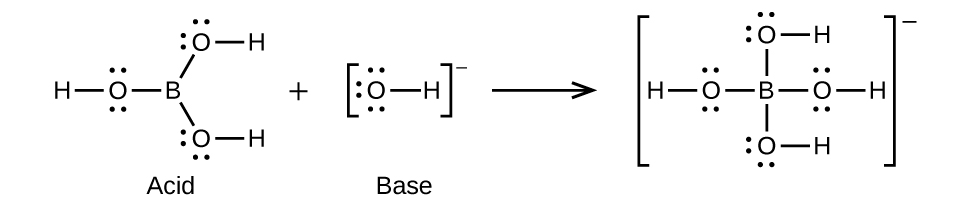
(c)
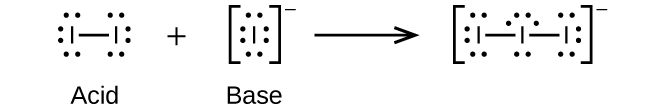
(d)
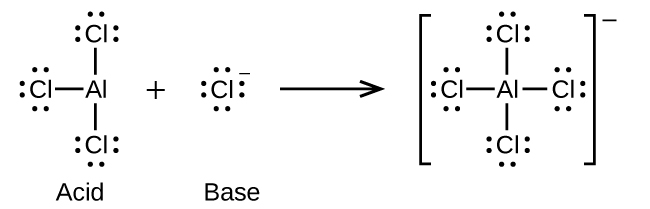
(e)
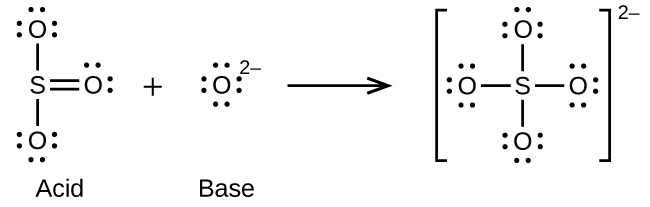


Feedback/Errata