27 Molecular Structure and Acid Strength (M15Q3)
Learning Objectives
- Describe the relationships between acid strength and molecular structure, and use them to predict the strength of an acid based on its molecular structure.
- Identify carboxylic acids and amines and their conjugate bases/acids.
| Key Concepts and Summary | Glossary | End of Section Exercises |
Effect of Molecular Structure on Acid-Base Strength
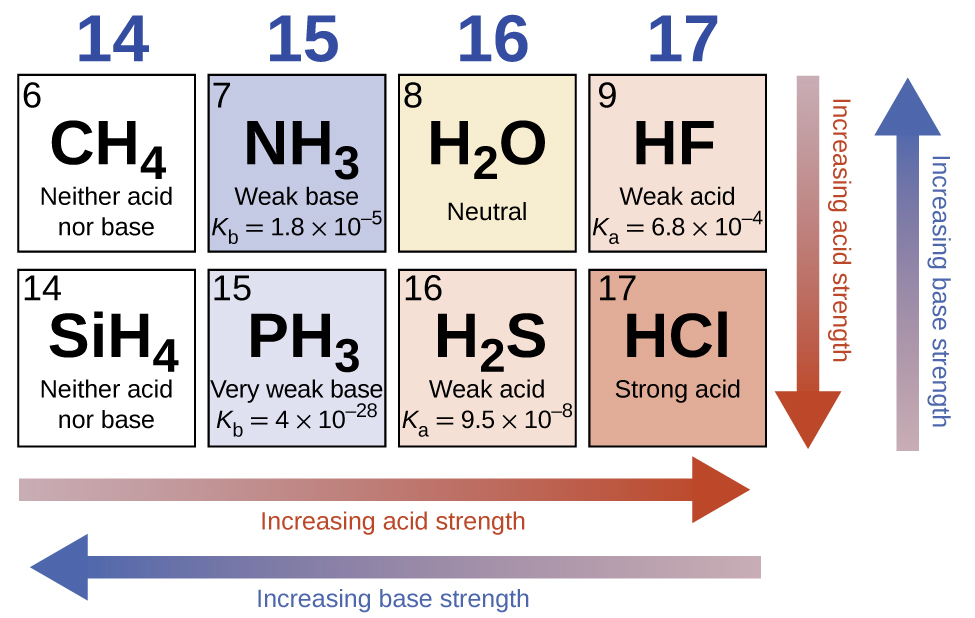
For most binary compounds of hydrogen with nonmetals, acidity increases as the H-A bond strength decreases (moving down in a group in the periodic table). For group 17 (or 7A depending on your periodic table), the order of increasing acidity is HF < HCl < HBr < HI. Likewise, for group 16 (or 6A), the order of increasing acid strength is H2O < H2S < H2Se < H2Te.
Across a row in the periodic table, the acidity of binary hydrogen compounds increases with increasing electronegativity of the nonmetal atom because the polarity of the H-A bond increases. Thus, the order of increasing acidity (for removal of one proton) across the second row is CH4 < NH3 < H2O < HF; across the third row, it is SiH4 < PH3 < H2S < HCl (see Figure 1).
Inorganic compounds containing oxygen and one or more hydroxyl (OH) groups tend to be acidic, depending on the position in the periodic table of the central atom E, the atom bonded to the hydroxyl group. Such compounds have the general formula OnE(OH)m, and include sulfuric acid, O2S(OH)2, sulfurous acid, OS(OH)2, nitric acid, O2NOH, perchloric acid, O3ClOH:
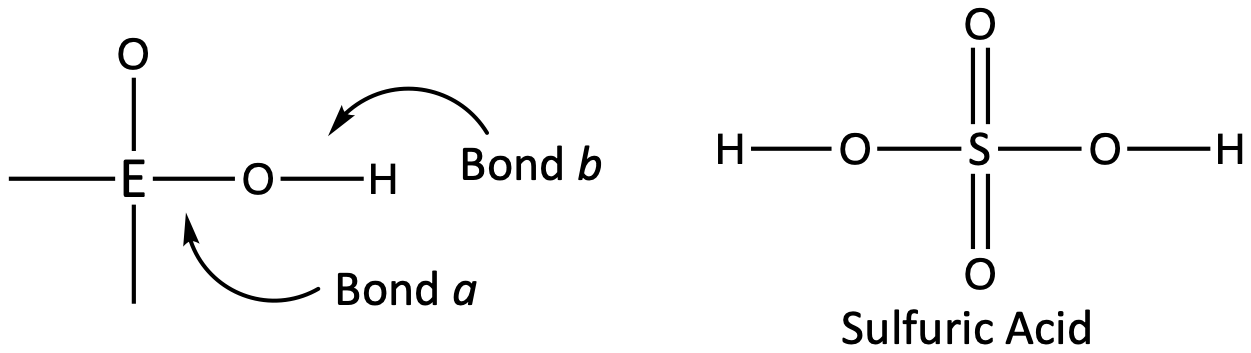
If the atom E has a relatively high electronegativity, it strongly attracts the electrons it shares with the oxygen atom, making bond a relatively strong. The oxygen-hydrogen bond, bond b, is thereby weakened because electrons are displaced toward E. Bond b is polar and readily releases hydrogen ions to the solution, so the material behaves as an acid. These compounds are commonly called oxyacids.
Key Concepts and Summary
In this section, we look at how to compare the strength of acids qualitatively for two classes of acids. The first are binary acids, where hydrogen is bonded to one other element (like HF, H2O, PH3). Within periods (or rows), the more electronegative the element is, the stronger the acid is due to the polarity of the bond created. The strongest acids are on the right of the periodic table. Within families (or columns), the larger the element is, the stronger the acid is due to a weaker bond created with larger atoms. The strongest acids are towards the bottom of the periodic table.
The second class of acids are oxyacids, which contain a hydrogen attached to an oxygen and other elements. Carboxylic acids are a common example of this type of acid. The strength of these acids are only determined by electronegativity. The more electronegative the elements are within the molecule, the stronger the acid. The increased polarity of the molecule weakens the O-H bond and allows the proton to dissociate more.
Even though we have focused primarily on the strength of acids in this section, the stronger the acid, the weaker the conjugate base after the proton dissociates. The weaker the acid, the stronger the conjugate base after the proton dissociates.
Glossary
binary compound
In this course, a compound with hydrogen and one other element
oxyacid
an inorganic compound that contains a O-H bond and other elements, such as a carboxylic acid
Chemistry End of Section Exercises
-
- Predict which acid in each of the following pairs is the stronger and explain your reasoning for each:
- H2O or HF
- HCl or HF
- HSO3– or HSO4–
- NH3 or H2S
- CH3COOH or CCl3COOH
- Predict which acid in each of the following pairs is the stronger and explain your reasoning for each:
Answers to Chemistry End of Section Exercises
- (a) HF, Both are in the same row so electronegativity is the greatest determining factor. F is more electronegative than O; (b) HCl, Both F and Cl are in the same group so size is the greatest determining factor. Cl is larger than F; (c) HSO4–, These are oxyacids so greater electronegativity of the central atom increases strength. A greater number of oxygens connected to the central atom increases electronegativity.; (d) H2S, Consider this in steps: Compare H2S to H2O, S is larger than O so H2S is a stronger acid. O is more electronegative than N so H2O is a stronger acid than NH3. Therefore, H2S is stronger than NH3. (e) CCl3COOH, The central atom is more electronegative with Cl atoms bounds to it rather than H atoms.


Feedback/Errata