26 Relative Strengths of Acids and Bases (M15Q2)
Learning Objectives
- Write balanced chemical equations for aqueous solutions of acids or bases.
- Recognize common strong and weak acids and bases.
- Predict relative acid or base strength using Ka and Kb values or other tabular data.
| Strengths of Acids and Bases | - Write equilibrium constant expressions for weak acids and bases (Ka, Kb)
- Write chemical equations for acid-base reactions and predict whether the reaction will be product-favored or reactant-favored at equilibrium based on Ka or Kb values.
| Key Concepts and Summary | Key Equations | Glossary | End of Section Exercises |
We can rank the strengths of acids by the extent to which they ionize in aqueous solution. A strong acid (HA) yields 100% (or very nearly so) of H3O+ and A− when the acid ionizes in water; Table 1 lists several strong acids. A weak acid gives small amounts of H3O+ and A−. The same is true of bases: strong bases dissociate 100% (or very nearly so).
| 6 Strong Acids | 7 Strong Bases |
| HCl hydrochloric acid | LiOH lithum hydroxide |
| HBr hydrobromic acid | NaOH sodium hydroxide |
| HI hydroiodic acid | KOH potassium hydroxide |
| HNO3 nitric acid | RbOH rubidium hydroxide |
| HClO4 perchloric acid | Ca(OH)2 calcium hydroxide |
| H2SO4 sulfuric acid | Sr(OH)2 strontium hydroxide |
| Ba(OH)2 barium hydroxide |
Strengths of Acids
The relative strengths of acids may be determined by measuring their equilibrium constants in aqueous solutions. In solutions of the same concentration, stronger acids ionize to a greater extent, and so yield higher concentrations of hydronium ions than do weaker acids. The equilibrium constant for an acid is called the acid-ionization constant, Ka. For the reaction of an acid HA:
we write the equation for the ionization constant as:
where the concentrations are those at equilibrium. The larger the Ka of an acid, the larger the concentration of H3O+ and A− relative to the concentration of the nonionized acid, HA. Thus a stronger acid has a larger ionization constant than does a weaker acid. The ionization constants increase as the strengths of the acids increase. (A table of ionization constants of weak acids appears in Appendix I.)
The following data on acid-ionization constants indicate the order of acid strength:
Another measure of the strength of an acid is its percent ionization. The percent ionization of a weak acid is the ratio of the concentration of the hydronium ion at equilibrium to the initial acid concentration, times 100:
Because the ratio includes the initial concentration, the percent ionization for a solution of a given weak acid varies depending on the original concentration of the acid, and actually decreases with increasing acid concentration. (Note that [H3O+]eq is calculated using an ICE table).
Strengths of Bases
We can rank the strengths of bases by their tendency to form hydroxide ions in aqueous solution. The reaction of a Brønsted-Lowry base with water is given by the following reactions:
Water is the acid that reacts with the base, HB+ is the conjugate acid of the base B, and the hydroxide ion is the conjugate base of water. A strong base yields 100% (or very nearly so) of OH− and HB+ when it reacts with water; Table 1 lists several strong bases. A weak base yields a small proportion of hydroxide ions. Soluble ionic hydroxides such as NaOH are considered strong bases because they dissociate completely when dissolved in water.

View the simulation of strong and weak acids and bases at the molecular level.
As we did with acids, we can measure the relative strengths of bases by measuring their base-ionization constant (Kb) in aqueous solutions. In solutions of the same concentration, stronger bases ionize to a greater extent, and so yield higher hydroxide ion concentrations than do weaker bases. A stronger base has a larger ionization constant than does a weaker base. For the reaction of a base, B or A-:
we write the equation for the ionization constant as:
where the concentrations are those at equilibrium. Again, we do not include [H2O] in the equilibrium expression because water is the solvent. The chemical reactions and ionization constants of the three bases shown are:
A table of ionization constants of weak bases appears in Appendix I. As with acids, percent ionization can be measured for basic solutions, but will vary depending on the base ionization constant and the initial concentration of the solution.
Relative Strengths of Acids and Bases
The extent to which an acid, HA, donates protons to water molecules depends on the strength of the conjugate base, A−, of the acid. If A- is a weak base, then the protons will not bind as strongly, relative to water, and the solution will contain more A- and H3O+ (stronger conjugate acid). If A− is a strong base, any protons that are donated to water molecules are recaptured by A−, and the solution will contain more HA and OH-. Thus there is relatively little A− and H3O+ in solution, and the acid, HA, is weak. If A− is a weak base, water binds the protons more strongly, and the solution contains primarily A− and H3O+—the acid is strong. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (Figure 1).
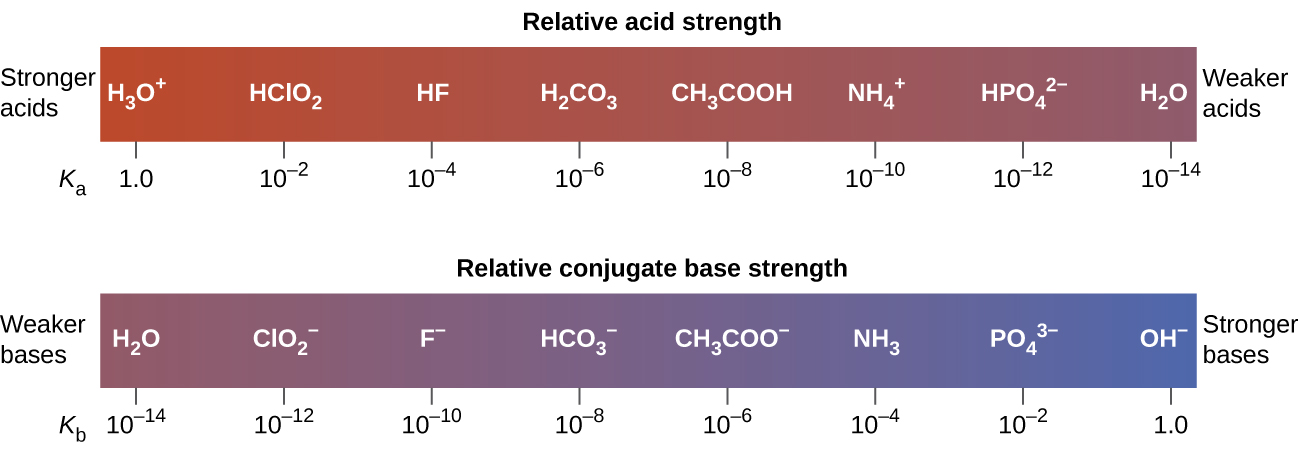
Figure 2 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. The acid and base in a given row are conjugate to each other.
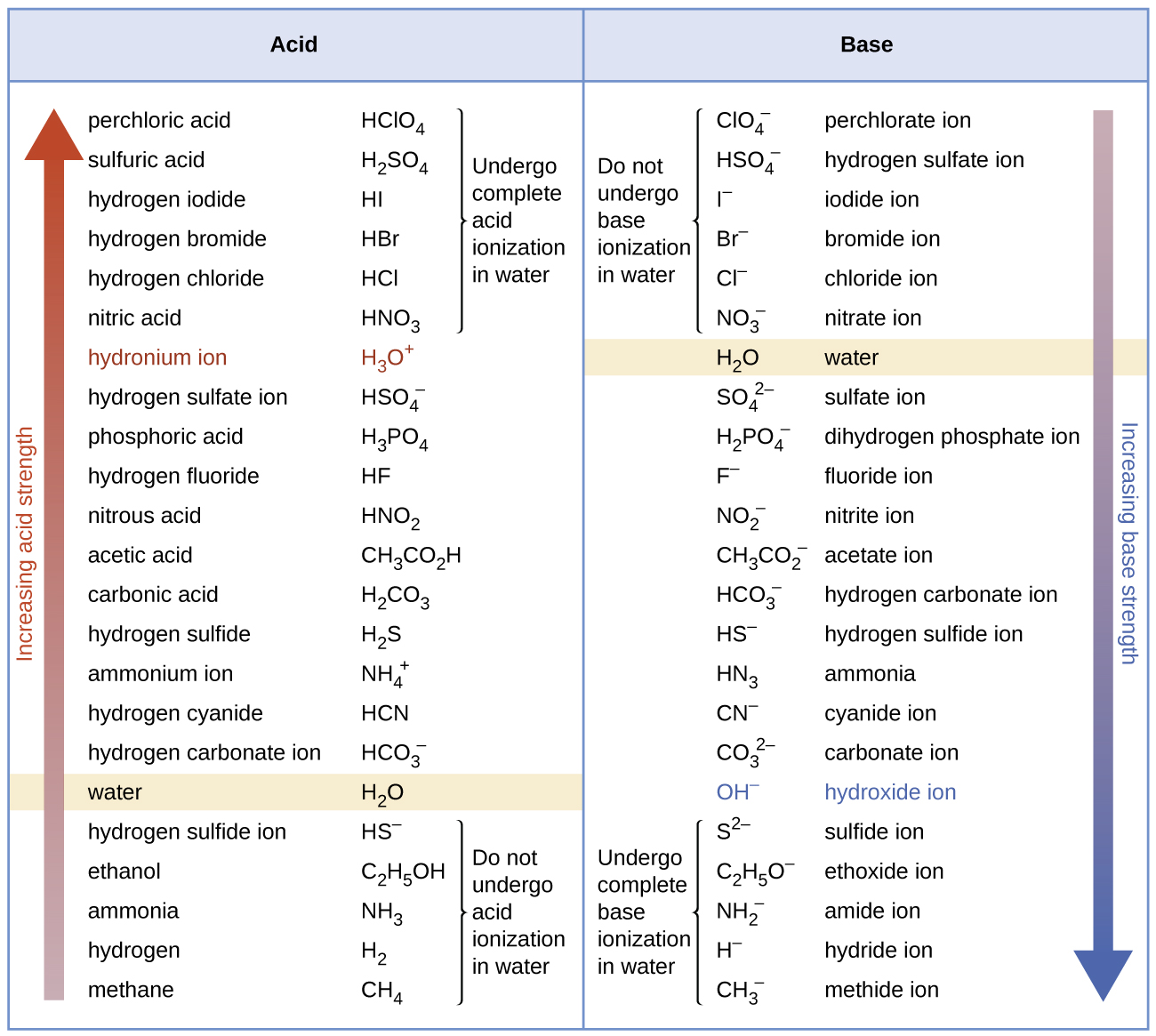
The first six acids in Figure 2 are the most common strong acids. These acids are completely dissociated in aqueous solution. The conjugate bases of these acids are weaker bases than water. When one of these acids dissolves in water, their protons are completely transferred to water, the stronger base.
Those acids that lie between the hydronium ion and water in Figure 2 form conjugate bases that can compete with water for possession of a proton. Both hydronium ions and nonionized acid molecules are present in equilibrium in a solution of one of these acids. Compounds that are weaker acids than water (those found below water in the column of acids) in Figure 2 exhibit no observable acidic behavior when dissolved in water. Their conjugate bases are stronger than the hydroxide ion, and if any conjugate base were formed, it would react with water to re-form the acid.
The extent to which a base forms hydroxide ion in aqueous solution depends on the strength of the base relative to that of the hydroxide ion, as shown in the last column in Figure 2. A strong base, such as one of those lying below hydroxide ion, accepts protons from water to yield 100% of the conjugate acid and hydroxide ion. Those bases lying between water and hydroxide ion accept protons from water, but a mixture of the hydroxide ion and the base results. Bases that are weaker than water (those that lie above water in the column of bases) show no observable basic behavior in aqueous solution.
Demonstration: Fizzing Races
Set up. The same amount of NaHCO3(s) is added to two flasks, one which contains HCl(aq) and the other HOAc(aq) (or CH3COOH). Both flasks contain the same volume and molarity of acid.
Prediction. Write the balanced reaction that occurs in each flask. Predict what will happen to the balloons both initially and at the end of each reaction.
Explanation: The reactions within the flasks are:
HCl(aq) + NaHCO3(s) → CO2(g) + H2O(l) + NaCl(aq)
CH3COOH(aq) + NaHCO3(s) → CO2(g) + H2O(l) + NaCH3COO(aq)
The reaction in the flask that contains HCl reacts very quickly, almost immediately inflating the balloon. The flask that contains CH3COOH reacts much more slowly, but ultimately inflates the balloon to the same volume.
HCl is a strong acid and is fully dissociated in solution. So all of the acidic protons (H+) are free to react immediately. CH3COOH is a weak acid and only partially dissociated in solution, which results in fewer H+ ions initially available to react. As CO2 is produced, the reaction continually shifts towards products and the CH3COOH ultimately fully reacts, but more slowly as the H+ ions are created gradually.
Key Concepts and Summary
Not all acids and bases donate and accept protons to the same extent. We find that 6 acids react to nearly 100% completion and classify these as strong acids. Likewise, 7 bases react to nearly 100% completion and we classify these as strong bases. For the simplicity of this course, all other acids and bases do not react 100% and reactants are always present at the end of the reaction. These molecules are classified as weak acids or bases and the Ka for the equilibrium reaction of the acid or base with water gives us insight into how much the acid or base reacts with water. The greater the Ka value, the stronger the acid. The greater the Kb value, the stronger the base.
Key Equations
- acid: HA(aq) + H2O(l) ⇌ H3O+(aq) + A-(aq) | Ka = [latex]\frac{[H_{3}O^{+}][A^{-}]}{[HA]}[/latex]
- base: B(aq) + H2O(l) ⇌ HB+(aq) + OH-(aq) | Kb = [latex]\frac{[HB^{+}][OH^{-}]}{[B]}[/latex]
- base: A-(aq) + H2O(l) ⇌ HA(aq) + OH-(aq) | Kb = [latex]\frac{[HA][OH^{-}]}{[A^{-}]}[/latex]
- percent ionization = [latex]\frac{[H_{3}O^{+}]_{eq}}{[HA]_{0}}[/latex] × 100
Glossary
acid-ionization constant, Ka
the equilibrium constant when an acid reacts with water to produce the conjugate base and hydronium ion
base-ionization constant, Kb
the equilibrium constant when a base reacts with water to produce the conjugate acid and hydroxide ion
Chemistry End of Section Exercises
- What compounds and ions are found in a solution of aqueous CH3COOH? Of aqueous HCl?
- Write a chemical equation to describe the proton transfer reaction that occurs when the following acids are added to water:
- HNO3
- PH4+
- H2S
- CH3CH2COOH
- CH3COOH
- H2PO4-
- HS-
- Write a chemical equation to describe the proton transfer reaction that occurs when the following bases are added to water:
- C5H5N
- (CH3)3N
- HS-
- H2PO3-
- CH3COO-
- NO2-
- NH3
- From the equilibrium concentrations given, calculate Ka for each of the weak acids and Kb for each of the weak bases.
- CH3COOH: [H3O+] = 1.34 × 10-3 M; [CH3COO-] = 1.34 × 10-3 M; [CH3COOH] = 9.855 × 10-2 M
- ClO-: [OH-] = 4.0 × 10-4 M; [HClO] = 2.38 × 10-5 M; [ClO-] = 0.273 M
- C6H5NH3+: [H3O+] = 2.3 × 10-3 M; [C6H5NH3+] = 0.233 M; [C6H5NH2] = 2.3 × 10-3 M;
- Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases, F− or CN−, is the stronger base? (See Figure 2).
- Write a chemical equation for the acid base reaction between F- and H2S. Will the reaction be reactant or product favored? (See Figure 2).
Answers to Chemistry End of Section Exercises
- CH3COOH: H2O, CH3COOH, H3O+, OH-, and CH3COO-;
HCl: H2O, H3O+, Cl-, and OH- -
- HNO3(aq) + H2O(l) ⇌ H3O+(aq) + NO3-(aq)
- PH4+(aq) + H2O(l) ⇌ H3O+(aq) + PH3(aq)
- H2S(aq) + H2O(l) ⇌ H3O+(aq) + HS-(aq)
- CH3CH2COOH(aq) + H2O(l) ⇌ H3O+(aq) + CH3CH2COO-(aq)
- CH3COOH(aq) + H2O(l) ⇌ H3O+(aq) + CH3COO-(aq)
- H2PO4-(aq) + H2O(l) ⇌ H3O+(aq) + HPO42-(aq)
- HS-(aq)+ H2O(l) ⇌ H3O+(aq) + S2-(aq)
-
- C5H5N(aq) + H2O(l) ⇌ OH-(aq) + C5H5NH+(aq)
- (CH3)3N(aq) + H2O(l) ⇌ OH-(aq) + (CH3)3NH+(aq)
- HS-(aq) + H2O(l) ⇌ OH-(aq) + H2S(aq)
- H2PO3-(aq) + H2O(l) ⇌ OH-(aq) + H3PO4(aq)
- CH3COO-(aq) + H2O(l) ⇌ OH-(aq) + CH3COOH(aq)
- NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)
- NH3(aq) + H2O(l) ⇌ OH-(aq) + NH4+(aq)
-
- Ka = 1.82 × 10-5
- Kb = 3.5 × 10-8
- Ka = 2.3 × 10-5
- CN-
- F-(aq) + H2S(aq) ⇌ HF(aq) + HS-(aq); reactant favored


Feedback/Errata