24 Types of Equilibria (Phase, Partition, Etc) (M14Q6)
Learning Objectives
- Relate various phase equilibria to features of a substance’s phase diagram.
Solid-Liquid & Liquid-Vapor Equilibria
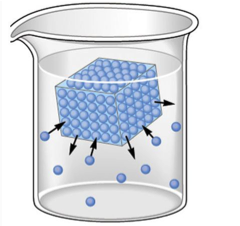
Solid-liquid and liquid-vapor equilibria exist during phase changes, as can be seen below in Figures 1 and 2. In the case of a solid-liquid equilibrium, the rate at which the solid phase is melting is equal to the rate at which the liquid phase is freezing:
H2O(s) ⇌ H2O(l)
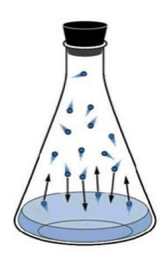
In the case of liquid-vapor equilibrium, the rate at which the liquid phase evaporates is equal to the rate at which the gas phase is condensing:
H2O(l) ⇌ H2O(g)
Condensed Phase Equilibria
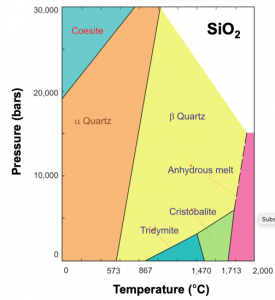
Condensed phase equilibria occur at the interface between two liquids or two solids. Examples of this are in the phase diagram of SiO2, along the black line between the orange and yellow shaded regions. At any temperature and pressure along this line, there will be an equilibrium between the α- and β- form of quartz, such that the rate of α-quartz converting into β-quartz is equal to the rate of β-quartz converting into α-quartz.
Solubility Equilibria
Solubility equilibria occur when a solid is dissolved into water. The equilibrium is between the undissolved solid phase and the dissolved aqueous phase of the solid. An example of this is adding glucose (sugar) to water, where so much sugar is added that not all of it dissolves, creating a saturated solution. The rate at which the glucose dissolves is equal to the rate at which the glucose precipitates:
glucose(s) ⇌ glucose(aq)
Partition Equilibria
Partition equilibria occur when a solute is dissolved in one liquid, a different liquid that is immiscible (will not mix) with the first liquid is added, the mixture is shaken, and the solute moves to the second liquid, as seen in Figure 4 below. Although the majority of the solute may end up in the second liquid, it is important to remember that this is an equilibrium, meaning that the solute will be present in both liquids, but perhaps in different quantities.
In this example, iodine is first dissolved in water. Hexane is carefully added to the system (left picture) and whole container is shaken to mix the liquids together (center picture) and create an equilibrium for iodine dissolved in both liquids:
I2(aq) ⇌ I2(hexane)
However, water and hexane are immiscible and the two liquids separate once the shaking is complete (right picture). Notice the difference between the left and the right pictures: the colors of the two liquids have changed, reflecting that iodine prefers to be dissolved in hexane. We will learn more about why this phenomenon occurs later in this course when we study intermolecular forces.

Key Concepts and Summary
In this section, we look at four types of equilibrium systems. All of these systems differ by which phases are in equilibrium with each other. A solid-liquid or liquid-vapor equilibrium are between the given phases. Condensed phase equilibria are between two of the same phases, either both liquid or both solid. Solubility equilibria have the same molecule as reactant and product, except the reactant is in the solid phase and the product are the dissolved aqueous ions. Partition equilibria again have the same molecule as reactant and product, except the molecule is dissolved in different liquids between the reactants and products.
Glossary
condensed phase equilibria
equilibrium system that occurs at the interface between two liquids or two solids
partition equilibria
equilibrium system that occurs when an immiscible liquid is added to another liquid with a dissolved solute. When the mixture is shaken, some of the solute moves from the first liquid to the added liquid.
solid-liquid, liquid-vapor equilibria
equilibrium system that occurs at the interface between a solid and liquid phase or a liquid and gas phase
solubility equilibria
equilibrium system that occurs when a solid is dissolved into water, solid ⇌ aqueous


Feedback/Errata