Unit Two
Day 12: VSEPR Theory, Hybrid Orbitals
As you work through this section, if you find that you need a bit more background material to help you understand the topics at hand, you can consult “Chemistry: The Molecular Science” (5th ed. Moore and Stanitski) Chapter 6-9 and 6-11 and Chapter 7-2e through 7-4, and/or Chapter 6.3-6.6 in the Additional Reading Materials section.
D12.1 VSEPR: Predicting Molecular Geometry
VSEPR theory predicts molecular geometry in the following steps:
- Write the Lewis structure of the molecule.
- Count the regions of electron density (lone pairs and bonds) around the central atom.
- Identify the electron-region geometry based on the number from (2).
- Use the number of lone pairs to determine the molecular geometry.
Example 1
Predicting Electron-region Geometry and Molecular Geometry: Lone Pairs on the Central Atom
Predict the electron-region geometry and molecular geometry of a water molecule.
Solution
The Lewis structure of H2O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two single bonds:
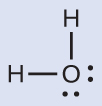
We predict that these four regions are arranged in a tetrahedral geometry. Thus, the electron-region geometry is tetrahedral and the molecular geometry is bent with an angle slightly less than 109.5°. In fact, the bond angle is 104.5°.
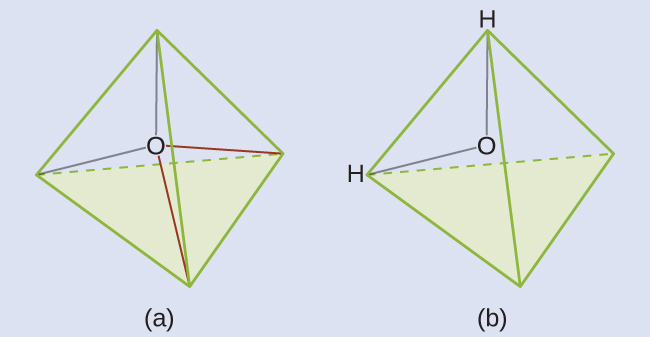
Check Your Learning
The hydronium ion, H3O+, forms when acids are dissolved in water. Predict the electron-region geometry and molecular geometry of this cation.
Answer:
electron-region geometry: tetrahedral; molecular geometry: trigonal pyramidal
Example 2
Predicting Electron-region Geometry and Molecular Geometry: SF4
Sulfur tetrafluoride, SF4, is extremely valuable for the preparation of fluorine-containing compounds used as herbicides. (SF4 is used as a fluorinating agent.)
Solution
The Lewis structure of SF4 indicates five regions of electron density around the sulfur atom: one lone pair and four single bonds:
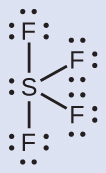
These five regions should adopt a trigonal bipyramidal electron-region geometry. To minimize lone pair repulsions, the lone pair occupies one of the equatorial positions. The molecular geometry is that of a seesaw.
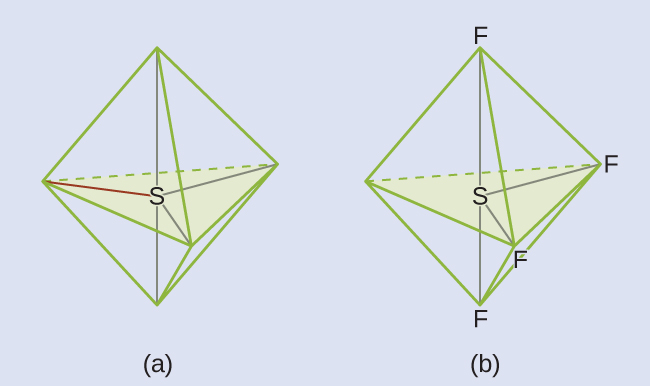
Check Your Learning
Predict the electron-region geometry and molecular geometry of XeF2.
Answer:
The electron-region geometry is trigonal bipyramidal. The molecular geometry is linear (because the lone pairs are all equatorial).
Example 3
Predicting Electron-region Geometry and Molecular Geometry: XeF4
Of all the noble gases, xenon is the most reactive, frequently reacting with elements such as oxygen and fluorine.
Solution
The Lewis structure of XeF4 indicates six regions of high electron density around the xenon atom: two lone pairs and four single bonds:
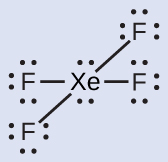
These six regions adopt an octahedral arrangement, which is the electron-region geometry. To minimize repulsions, the lone pairs should not be at the smallest angle (90°). The five atoms are all in the same plane and have a square planar molecular geometry.
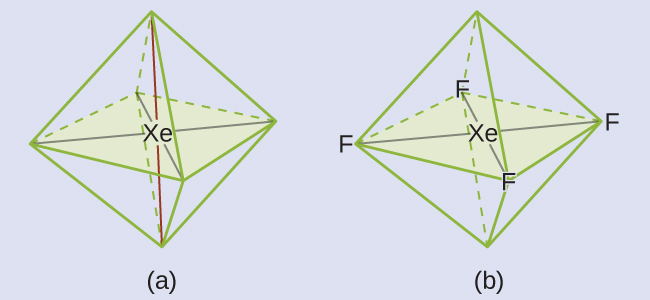
Check Your Learning
In a certain molecule, the central atom has three lone pairs and two single bonds. What will the electron-region geometry and molecular geometry be?
Answer:
electron-region geometry: trigonal bipyramidal; molecular geometry (molecular structure): linear
Exercise 1: Predicting Molecular Geometry
VSEPR enables easy prediction of molecular geometries from Lewis structures, but keep in mind that VSEPR theory only considers electron-pair repulsions. All other relevant interactions, such as nuclear-nuclear repulsions and nuclear-electron attractions, are ignored. Consequently, VSEPR can yield a misleading picture of how lone pairs are arranged, especially when there are multiple lone pairs, on an atom. It also fails to predict correctly the geometry of transition metal complexes.
D12.2 Molecular Structure for Multicenter Molecules
Larger molecules do not have a single central atom, but are connected by a chain of interior atoms that each possess a “local” geometry. The way these local structures are oriented with respect to each other influences the overall molecular shape.
Example 4
Predicting Structure in Multicenter Molecules
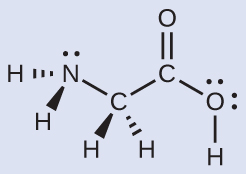
The Lewis structure for the simplest amino acid, glycine, H2NCH2CO2H, is shown here. Predict the local geometry for the nitrogen atom, the two carbon atoms, and the oxygen atom with a hydrogen atom attached:
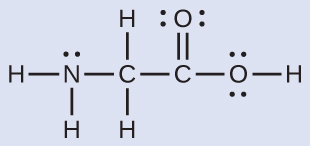
Solution
The structure has four central atoms. From left to right in the structure above they are N, C, C, and O. Click the structure below to see a 3D manipulable model.
Use VSEPR for each central atom independently. The electron-region geometries are
- nitrogen––four regions of electron density; tetrahedral
- carbon (CH2)––four regions of electron density; tetrahedral
- carbon (COOH)—three regions of electron density; trigonal planar
- oxygen (OH)—four regions of electron density; tetrahedral
The local structures are
- nitrogen––three single bonds, one lone pair; trigonal pyramidal
- carbon (CH2)—four single bonds, no lone pairs; tetrahedral
- carbon (COOH)—three bonds (one double and two single), no lone pairs; trigonal planar
- oxygen (OH)—two single bonds, two lone pairs; bent
Check Your Learning
Another amino acid is alanine, which has the Lewis structure shown here. Predict the electron-region geometry and local structure of the nitrogen atom, the three carbon atoms, and the oxygen atom with hydrogen attached. Draw a valid wedge-dash structure for alanine.
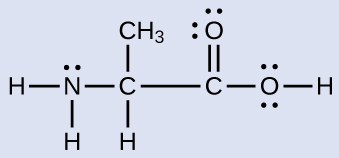
Answer:
Electron-region geometries: nitrogen—tetrahedral; carbon (CH)—tetrahedral; carbon (CH3)—tetrahedral; carbon (COOH)—trigonal planar; oxygen (OH)—tetrahedral.
Local structures: nitrogen—trigonal pyramidal; carbon (CH)—tetrahedral; carbon (CH3)—tetrahedral; carbon (COOH)—trigonal planar; oxygen (OH)—bent.
Click here to see a 3D molecular model. Make certain that the wedge-dash structure you drew agrees with the model. The model does not allow rotation around single bonds, so you may need to imagine how rotation around single bonds would affect the appearance of the structure.
Example 5
Molecular Simulation
For a few molecules the molecular shape simulator allows you to choose a molecular formula and see the molecular shape in 3D You can also construct a triatomic molecule with different types of bonds, show idealized bond angles and lone pairs by checking or unchecking the boxes under “Options” on the right, and also use the “Name” checkboxes at bottom-left to display or hide the electron-region geometry (called “electron geometry” in the simulator) and/or molecular geometry (called “molecular shape” in the simulator).
Build the molecule HCN in the simulator based on the following Lewis structure:
H-C≡N:
Click on each bond type or lone pair at right to add that group to the central atom. Once you have the complete molecule, rotate it to examine the predicted molecular structure. What molecular structure is this?
Solution
The molecular structure is linear.
Check Your Learning
Build a more complex molecule in the simulator. Identify the electron-region geometry, molecular geometry, and bond angles. Then try to find a chemical formula that would match the structure you have drawn.
Answer:
Answers will vary. For example, an atom with four single bonds, a double bond, and a lone pair has an octahedral electron-region geometry and a square pyramidal molecular geometry. XeOF4 is a molecule that adopts this structure.
D12.3 Chiral Molecules
Molecules with the same connectivity but different arrangements of the atoms in space are called stereoisomers. Geometric (cis-trans) isomers (see Section 8.5) are one type of stereoisomer.
Another type of stereoisomer is a pair of molecules that are mirror images that cannot be superimposed on each other. Such stereoisomers are called enantiomers (or optical isomers). These molecules are described as chiral (from the Greek word cheir, χειρ, meaning “hand”). Two substances that are enantiomers have identical physical properties but their chemical and physiological properties may differ. (An infamous example of different chemical and physiological properties involves the drug thalidomide.) Your hands, feet, and ears are examples of enantiomers. Your left and right hands are mirror images of each other, but you cannot superimpose them. Try putting your right glove on your left hand—it just doesn’t work.
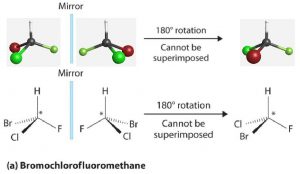
Most chiral molecules have at least one atom that is bonded to four different groups. For example, bromochlorofluoromethane (Figure 1) is not superimposable on its mirror image; it and its mirror image molecule are a pair of enantiomers. The carbon atom marked by an asterisk is a chiral center and is referred to as an asymmetric carbon atom or a chiral carbon atom.
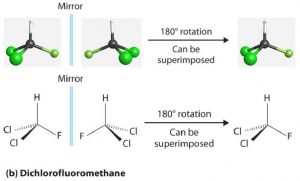
If the bromine atom is replaced by a second chlorine atom (making dichlorofluoromethane), the molecule and its mirror image are superimposable. Thus dichlorofluoromethane is not a chiral molecule (it is achiral). Notice that its central carbon is bonded to two groups that are the same (two Cl atoms).
In most cases, the easiest way to figure out whether a molecule is chiral is to look for one or more chiral atoms—the general rule is that molecules with at least one chiral atom are chiral, and molecules with no chiral atoms are achiral. Just because you see dashed and solid wedges in a structure, do not automatically assume that you are looking at a stereocenter. For example, lactic acid has an asymmetric (chiral) carbon atom (denoted by *) and is a chiral molecule, while isobutyric acid is not a chiral molecule.
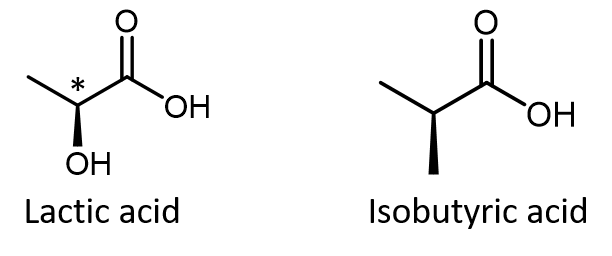
Make sure that you can recognize from the wedge-dash Lewis structure that the chiral carbon atom in lactic acid has four different groups around it but the corresponding carbon atom in isobutyric acid does not. Use these three links to access 3D rotatable models of the two enantiomers of lactic acid and the single structure of isobutyric acid. Lactic acid link 1. Lactic acid link 2. Isobutyric acid.
D12.4 Orbital Hybridization
While VSEPR helps us to predict molecular geometry, it provides no information on the orbitals occupied by bonding and lone-pair electrons in a molecule. Such information is important for understanding molecular properties and chemical reactivity. Molecular orbital theory provides all the information we need, but it is often difficult to interpret MOs that are delocalized over an entire molecule. Valence bond theory focuses on how the atomic orbitals combine to give individual chemical bonds and uses the extent of overlap of the orbitals to infer strength of chemical bonds. Orbitals that overlap more form bonds that are stronger than those that have less overlap.
In valence bond theory, some or all of an atom’s atomic orbitals are combined to form new orbitals with different shapes that allow for greater overlap with orbitals on other atoms. Combining orbitals on the same atom is called orbital hybridization and the new orbitals that result are called hybrid orbitals. Unlike an atomic orbital, a hybrid orbital has greater electron density on one side of an atom than the other side. This greater electron density provides better overlap with an orbital from another atom when forming a σ bond.
Hybridization combines the wave functions for two or more orbitals on the same atom to give two or more new orbitals. For example, consider combining a single valence s orbital with a single valence p orbital. The s orbital is spherically symmetric so it has the same phase (mathematical sign) on either side of the nucleus, but the p orbital changes sign at the nucleus. Thus, on one side of the nucleus the wave functions for the s and p orbitals are in phase and on the other side they are out of phase. If we combine the two wave functions by adding them, the new function will be larger on the side where the waves are in phase and smaller where the waves are out of phase. If we subtract the p-orbital wave function from the s-orbital wave function, the resultant wave will be larger on the side of the atom where the waves are out of phase and smaller where they are in phase.
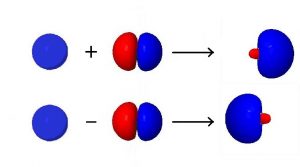
These ideas are important in understanding hybridization:
- Hybrid orbitals are unnecessary in isolated atoms. Hybrid orbitals result in stronger bonding between covalently bonded atoms.
- In a set of hybrid orbitals, the number of hybrid orbitals equals the number of atomic orbitals that were combined to produce the hybrid orbitals.
- The type of hybrid orbitals for a bonded atom correlates with the local geometry of that atom, which usually can be determined using VSEPR.
- Hybrid orbitals overlap to form σ bonds. Unhybridized atomic orbitals overlap to form π bonds.
D12.5 Types of Hybrid Orbitals
sp Hybridization
Combining the valence s AO with one of the valence p AOs yields two degenerate sp hybrid orbitals oriented at 180° to each other. This is the same orientation predicted by VSEPR for two regions of electron density. For example, the beryllium atom in the linear BeCl2 molecule has sp hybridization, as shown in Figure 3.
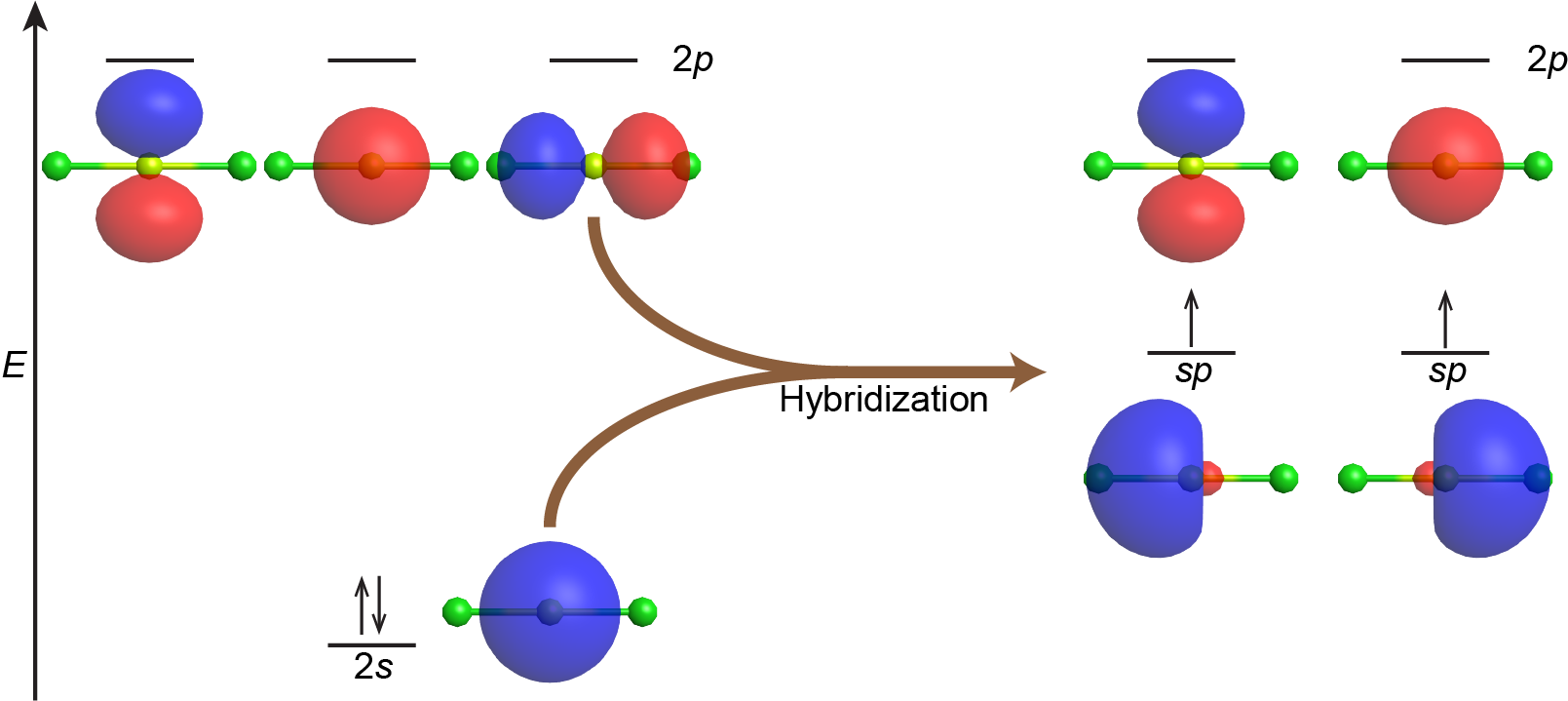
Figure 3 also illustrates the electronic differences between an isolated vs a bonded Be atom. The sp orbital is higher in energy than the 2s AO and lower in energy than the 2p AO. We can see that the hybridized orbitals would not be energetically favorable for an isolated Be atom—the atomic orbitals are optimal. Hence, hybridized orbitals are only useful in covalently bonded atoms.
The unpaired electron in each sp orbital pairs up with an unpaired electron from a p orbital on a Cl atom to form a Be-Cl σ bond. The 180º orientation of the two sp hybrid orbitals yields a linear geometry for BeCl2, the same as predicted using VSEPR.
sp2 Hybridization
Combining one valence s orbital and two valence p orbitals produces three degenerate sp2 hybrid orbitals. These are oriented toward the corners of an equilateral triangle—a trigonal planar geometry. For example, VSEPR predicts that the boron atom in the BF3 molecule has trigonal planar geometry. This corresponds to sp2 hybridization and the B atom is involved in three σ bonds to fluorine atoms. The orbitals and electron distribution in an isolated B atom and in the bonded B atom in BF3 are illustrated in Figure 4.
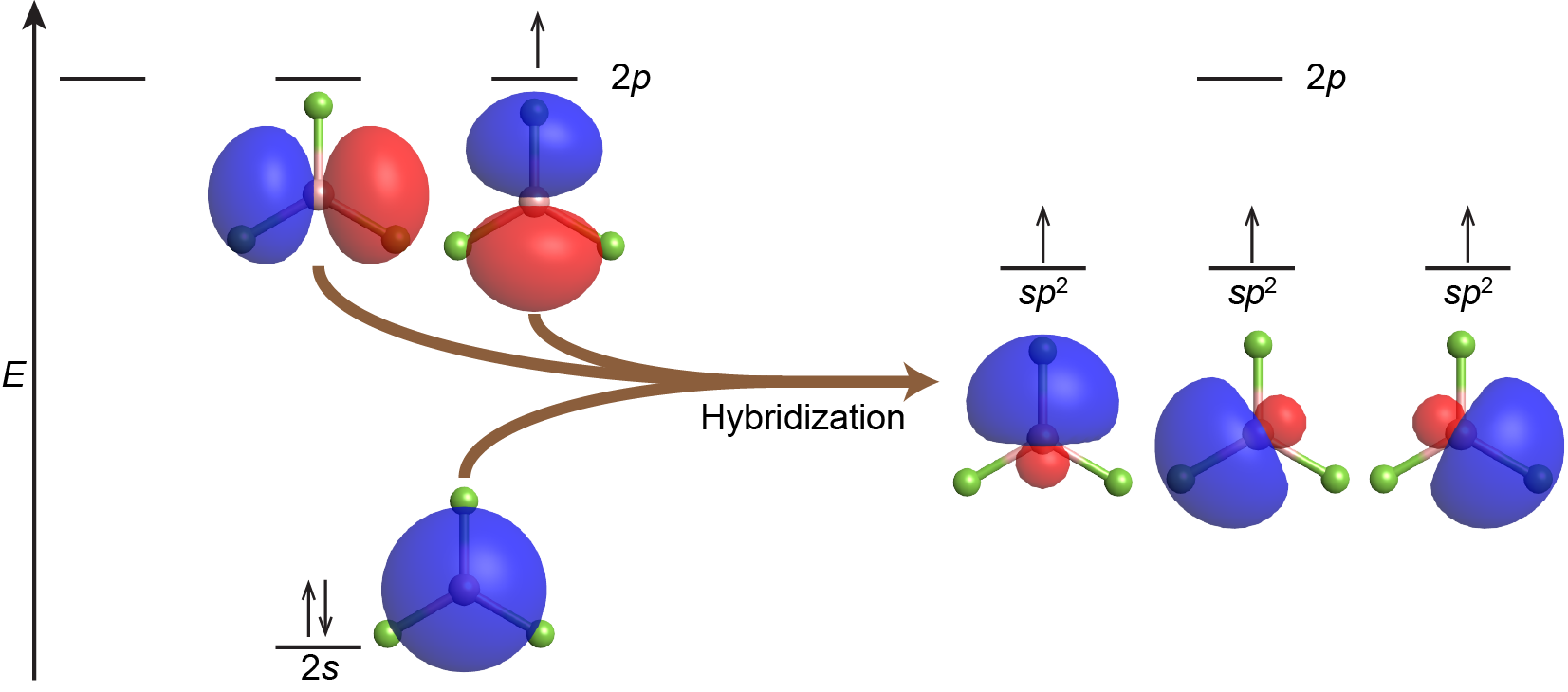
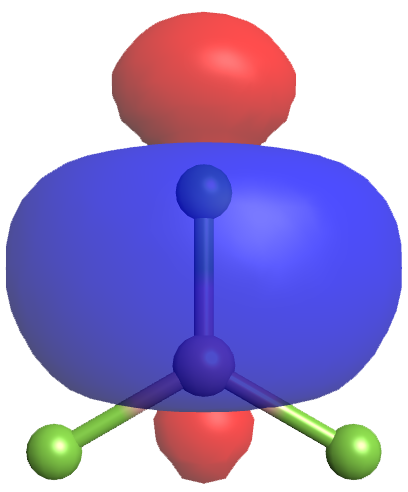
Each of the unpaired electrons in an sp2 orbital can pair with an unpaired electron in a p orbital on a fluorine atom to form a B-F σ bond (Figure 5). The in-plane 120º orientation of the three sp2 hybrid orbitals corresponds to a trigonal planar geometry for BF3, as predicted by VSEPR.
sp3 Hybridization
The mixing of one s orbital and all three p orbitals produces four degenerate sp3 hybridized orbitals, each pointed towards a different corner of a tetrahedron. For example, the carbon atom in methane, CH4, exhibits sp3 hybridization. The orbitals and electron distribution in an isolated C atom and in the bonded C atom in CH4 is illustrated in Figure 6.
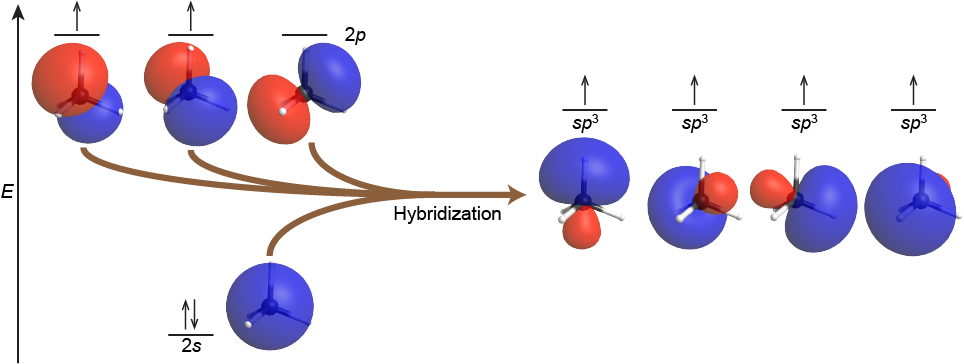
In the methane molecule, each of the four H 1s orbitals overlaps with one of the four C sp3 orbitals, two electrons pair up, and a C-H σ bond forms. This results in four strong covalent C-H bonds at angles of 109.5°: the tetrahedral CH4 molecule.
Electron Lone Pairs
A hybrid orbital can also hold a lone pair instead of being involved in σ bonding. For example, the nitrogen atom in ammonia, NH3, is surrounded by three bonding electron pairs and a lone pair of electrons directed to the four corners of a tetrahedron. The nitrogen atom is sp3 hybridized with one hybrid orbital occupied by the lone pair, corresponding to its tetrahedral electron-region geometry predicted by VSEPR. This results in a trigonal pyrimidal molecular structure.
D12.6 Double and Triple Bonds
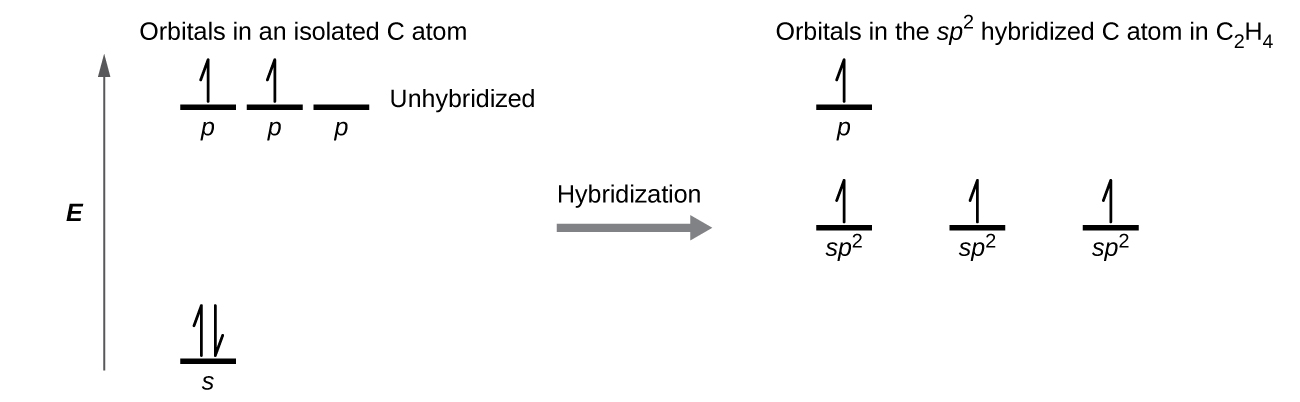
The Lewis structure of ethene, C2H4, shows us that each carbon atom is surrounded by one other carbon atom and two hydrogen atoms. VSEPR predicts a trigonal planar electron-region geometry.
Each carbon atom is sp2 hybridized. The three sp2 orbitals on each carbon form two C–H σ bonds and a σ bond between the two C atoms. Figure 7 shows the hybridization.The π bond in the C=C double bond results from the overlap of the unhybridized 2p orbital on each carbon atom.
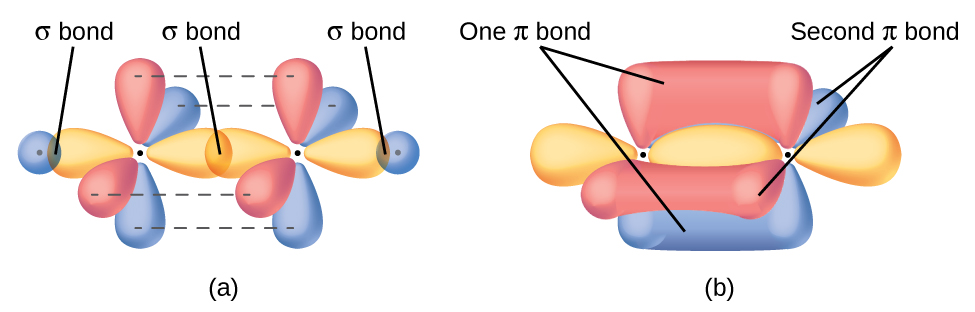
Each unhybridized 2p orbital is perpendicular to the plane of the sp2 hybrid orbitals (Figure 8). Thus when the p orbitals overlap in a side-by-side fashion to form a π bond, the electron densities in the π bond are above and below the plane of the σ bonding system.

If the two planes of sp2 hybrid orbitals (from the two carbon atoms) are tilted relative to each other, the 2p orbital from one carbon would be tilted relative to the 2p orbital on the other carbon atm. The p orbitals would no longer be able to overlap as much and the π bond would be weaker. This is a significant difference between σ and π bonds: one atom rotating around the internuclear axis with respect to the other atom does not change the extent to which the σ bonding orbitals overlap because the bonding electron density is symmetric about the bond axis; in contrast, rotation by 90° about the internuclear axis would break the π bond. because the p orbitals would no longer overlap. This is the reason cis-trans (geometric) isomers (Section 8.4) can be isolated as separate compounds at room temperature.
In acetylene, H−C≡C−H, each carbon atom is sp hybridized with two unhybridized 2p orbitals. One sp hybrid orbital from each C atom overlaps end-to-end to form a C-C σ bond, the other sp orbitals form C-H σ bonds with hydrogen atoms, and the unhybridized 2p orbitals overlap side by side to form two perpendicular C-C π bonds (Figure 9). The two carbon atoms of acetylene are thus bound together by one σ bond and two π bonds, giving a triple bond.
Exercise 2: Identifying Hybridization
Because π bonds are formed from unhybridized atomic orbitals, for molecules that have two or more major resonance structures that indicate contributions from various arrangements of π bonds, the assignment of hybridization is similar to the process discussed above. However, keep in mind that atoms do not move between resonance structures, so the hybridization (and molecular geometry) assigned to one resonance structure must be similarly assigned to all other resonance structures of the same molecule. For example, the nitrogen atom in formamide has a sp2 hybridization.
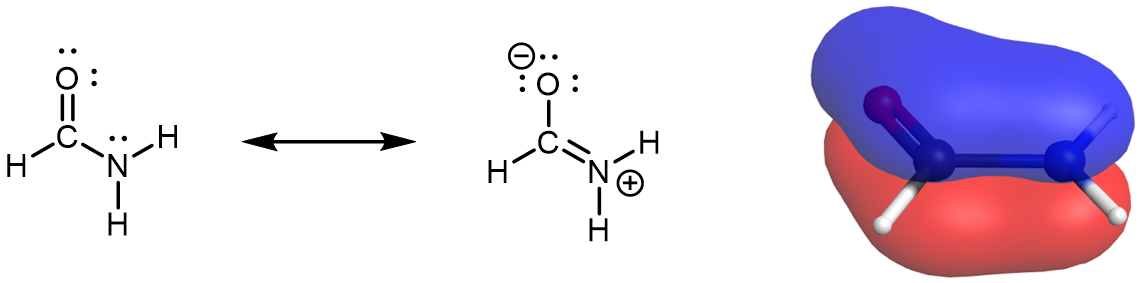
There is an unhybridized 2p orbital on the N atom that participates in the π bonding network described by the set of resonance structures. Therefore the nitrogen atom must have a trigonal planar geometry; it does not oscillate between trigonal pyramidal and trigonal planar. This link to a rotatable structure of formamide shows that the molecule is planar.
Example 6
Assignment of Hybridization Involving Resonance Structures
Some acid rain results from the reaction of sulfur dioxide with atmospheric water vapor, followed by the formation of sulfuric acid. Sulfur dioxide, SO2, is a major component of volcanic gases as well as a product of the combustion of sulfur-containing coal. What is the hybridization of the S atom in SO2?
Solution
The resonance structures of SO2 are
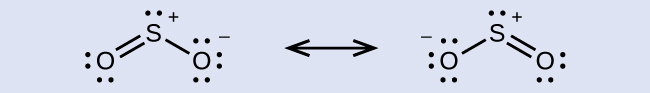
In either resonance structure, the sulfur atom has one single bond, one double bond, and one lone pair. Therefore, the electron-region geometry is trigonal planar, and the hybridization of the sulfur atom is sp2. The lone pair resides in one of the sp2 orbitals, and the unhybridized p orbital is involved in the delocalized π bonding.
Check Your Learning
Another acid in acid rain is nitric acid, HNO3, which is produced by the reaction of nitrogen dioxide, NO2, with atmospheric water vapor. What is the hybridization of the nitrogen atom in NO2? (Note: the lone electron on nitrogen occupies a hybridized orbital just as a lone pair would.)
Answer:
sp2
Podia Question
Ozone in the stratosphere protects Earth’s surface from ultraviolet radiation, which made the hole in the ozone layer over Antarctica a serious concern. Ozone close to Earth’s surface is formed by chemical reactions among pollutants emitted by automobiles and can make bronchitis, emphysema, and asthmas worse. Consider the ozone molecule , O3.
1. Write a Lewis structure for ozone; include all resonance structures connected by double arrows.
2. Predict the shape of the ozone molecule and the approximate bond angle. Explain how you predicted the shape.
3. Based on the delocalization represented in the resonance structures, draw a diagram showing the π bonding MO in the ozone molecule. Also, describe the π bonding MO in words.
4. Which atomic orbitals contribute to the π bonding MO? For each AO, describe the type of AO and its location (which O atom).
5. Determine the hybridization of the central oxygen atom in ozone. Can the terminal oxygen atoms have sp3 hybridization? Why or why not?
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.