D5.2 Metals
Based on iron’s boiling point of 3135 K (2862 °C), the forces between iron atoms must be much greater than just LDFs. What you have already learned about atomic structure, effective nuclear charge, and valence electrons can be used to make sense of the much larger forces that attract iron atoms, as well as atoms of other metals.
Metals, which include most of the elements in the periodic table (figure below), have characteristic macroscopic properties: they conduct heat and electricity well when they are solids or liquids; they have lustrous (metallic-looking) solid surfaces; and they deform, rather than shatter, when hammered (they are malleable). Many metals are hard, quite strong, and useful as construction materials. What gives rise to these characteristics?
Metallic substances are made of atoms whose valence electron(s) can be removed with relatively low quantities of energy, that is, elements with relatively low ionization energies. Valence electrons of metals experience lower effective nuclear charge than valence electrons of nonmetals. Hence, metals tend to form positive ions and nonmetals tend to form negative ions.
In a metallic solid, atoms are packed closely (Figure: Models of Solid Copper). One model describes solid metals at the atomic scale as atoms surrounded by mobile valence electrons—a so-called “sea” of electrons. Each “atom” consists of the nucleus and core electrons; that is, a positively charged ion that has lost all of its valence electrons. The loosely-bound valence electrons are shared (delocalized) among many different atoms. Coulombic attractions between the sea of shared valence electrons and the metal ions is known as metallic bonding. The strength of metallic bonding increases as the number of valence electrons shared in the electron sea increases.
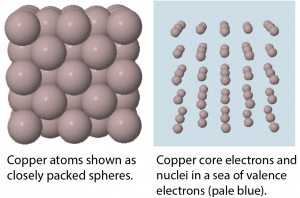
Because the valence electrons are delocalized over many metal atoms, if one atom moves relative to others, the attractions between valence electrons and atomic cores remain, that is, metallic bonding is not specific to a particular direction. This characteristic accounts for many of the observed physical properties of metals.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

