D26.3 Fuels
Consider some examples of fuels that you may have used recently. Natural gas for a kitchen stove or electric power plant is mainly methane. LP (liquefied petroleum) gas used for gas grills and for homes not connected to natural gas pipelines is mainly propane and butane. Gasoline and diesel fuel, used to power automobiles, trucks, and trains, consists of a complex mixture of hydrocarbons, mainly alkanes. Coal, which is burned in power plants to generate electricity, is primarily carbon, with a mixture of other elements as minor constituents. These various fuels all have at least one thing in common: they react with oxygen to form carbon dioxide and (in most cases) water with a very large decrease in Gibbs free energy. That is, in the presence of oxygen, which is available everywhere on earth in the air, these fuels are all thermodynamically unstable compared to CO2 and H2O.
Consider what fuels are used for. Gasoline is used to increase a car’s kinetic energy as the car accelerates. Electricity from a coal-fired or natural gas-fired power plant can be used to power a motor. Both of these uses of fuels involve work: a process that transfers kinetic energy to or from a macroscopic object. It turns out that the large, negative Gibbs free energy change when a thermodynamically unstable fuel reacts is necessary to do that work. The negative of ΔrG is the maximum work that can be done when a spontaneous process occurs. That is,
−ΔrG = w
where w is the maximum work that can be done on the surroundings (to accelerate the automobile, for example). If ΔrG is negative, as in the case of the fuels we mentioned, then the maximum work done on the surroundings is positive. If ΔrG is positive then work is required to cause the chemical reaction to happen (and none of the reactants is a fuel). In this latter case, w is interpreted as the minimum work required to cause the (non-spontaneous) reaction to occur. (Such non-spontaneous reactions are involved in recharging batteries, for example, a subject we will discuss later.)
A second major requirement for a fuel is that it must react when we want it to, but not when we don’t. Translating that to chemical terms, the reactants in the fuel’s spontaneous reaction must be kinetically metastable relative to the products of the reaction. For example, consider the combustion of propane in LP gas to form carbon dioxide and water:
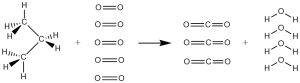
It is clear that for this reaction to occur, the bonds in the reactants must all be broken: there are no C-H, C-C or O=O bonds in the products. This bond breaking need not all take place at once, but somehow the process of breaking bonds must be started. The bond energies for reactants and products are
| Reactants | |
| C-H | 416 kJ/mol |
| C-C | 346 kJ/mol |
| O=O | 498 kJ/mol |
| Products | |
| C=O | 803 kJ/mol |
| O-H | 467 kJ/mol |
There are 15 bonds in the reactants and only 14 bonds in the products, but the product bonds are very strong so the reaction has a negative standard Gibbs free energy change. (You can verify this by calculating ΔrG° from thermodynamic data in the appendix.; ΔrG° = −2074 kJ/mol.) However, the reactants have no functional groups where reaction might begin, and the weakest bond in the reactants, the C-C bond, is still pretty strong at 346 kJ/mol. Thus it is likely that whatever process initiates the reaction will have quite a large energy barrier. A large activation energy barrier implies a slow reaction or perhaps no reaction at room temperature. Therefore the reactants are kinetically metastable relative to the products at room temperature.
Other requirements for a fuel are that it is available in large supply at the surface of Earth, or that it can be made from something that is readily available. Fossil fuels, such as the coal, natural gas, and gasoline that we have been discussing are available, although it sometimes takes a lot of technology (fracking or underwater drilling) to get them. Another fuel that is not used much at present, but might be in the future is hydrogen, which could be produced inexpensively using electricity generated by solar panels to electrolyze water into hydrogen and oxygen.
Activity: Is Ammonia a Possible Fuel?
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

