D7.1 Bond Length and Bond Energy
Formation of a stable covalent molecule involves sharing electrons between two or more nuclei (the electrons occupy molecular orbitals that increase electron density between nuclei). The bond length between any two adjacent nuclei in such a covalent molecule is the distance between the two nuclei at the minimum energy in a graph of energy versus nuclear separation. For example, the bond length in a H2 molecule is 74 pm, as shown in this figure.
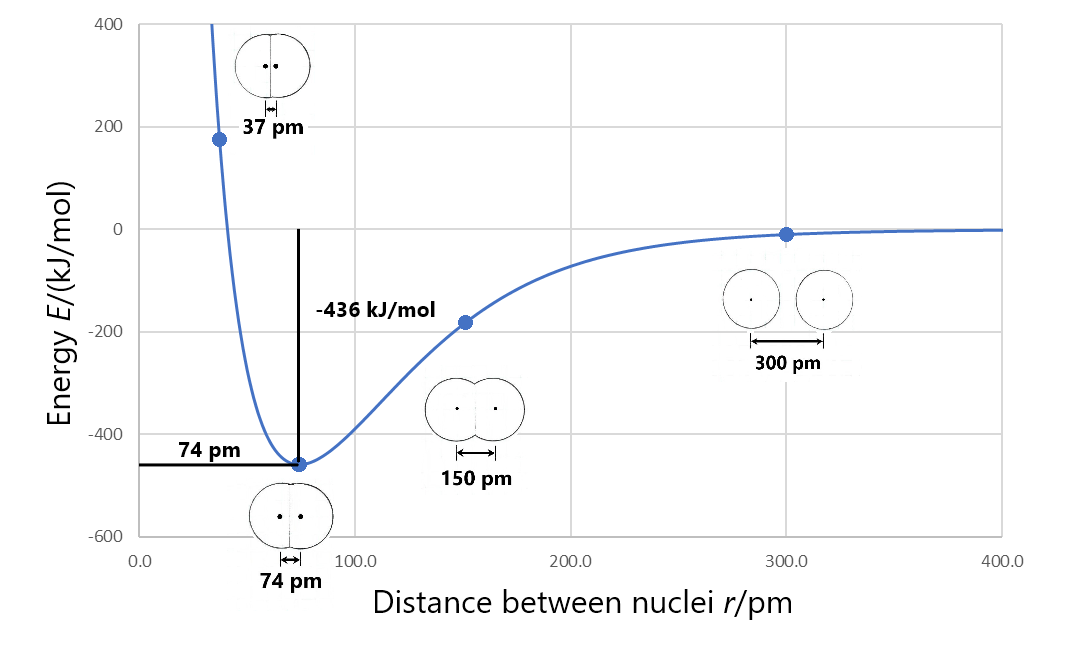
Bond energy is the energy change when a chemical bond is broken; that is, when two bonded atoms are completely separated. For example, the figure above shows that the bonded hydrogen atoms have energy of −436 kJ/mol relative to the separated hydrogen atoms. This means that the energy of the molecule must be increased by 436 kJ/mol to separate the atoms (break the bond). So the bond energy for H2 is 436 kJ/mol.
Exercise: Bond Energy
Lengths of single bonds can be roughly estimated by using the atomic radii of the bonded atoms. For example, adding the atomic radius (also called covalent radius) of C (77 pm) to that of O (74 pm), estimates the length of a C—O bond to be 151 pm. This is quite close to the average C—O bond length of 143 pm. (Both of these values are estimates because covalent radii and bond lengths are averaged over many molecules and therefore are not exact for any specific molecule.)
In general, the bigger the atoms are, the longer the bond between them is. For example, consider the following trend in the average C-X bond lengths, where X is a halogen:
| C-F | 141 pm |
| C-Cl | 176 pm |
| C-Br | 191 pm |
| C-I | 210 pm |
Bond lengths are also dependent on bond order. For example, the C—C single bond has an average length of 154 pm, while a C=C double bond is 134 pm long, and a C≡C triple bond has an average length of 121 pm.
Exercise: Bond Length and Atomic Radius
Comparisons of the average bond length and bond energy values show a general trend: a covalent bond with a shorter bond length usually has a larger bond energy.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

