D29.6 Activation Energy, Concentration, and Reaction Rate
We have now developed the ideas needed to predict the rate of a reaction at various values of concentration and temperature. The Arrhenius equation enables calculation of the rate constant at a given temperature. The rate law enables calculation of the rate, given k and concentrations of species that affect the rate. The previous section outlined one way to determine the rate law. In this section we illustrate how to experimentally determine the activation energy.
Exercise: Calculating Rate Constants
If we take the natural logarithm of both sides of the Arrhenius equation, we have:
which has a standard linear equation format:
![Rendered by QuickLaTeX.com \begin{array}{rcccl} \text{ln}(k) & = & \left(-\dfrac{E_a}{R}\right)\left(\dfrac{1}{T}\right) &+& \text{ln}(A) \\[0.5em] y &=& m\ \ \ \ x &+& b \end{array}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-82d0714e5f373e9c36439d97965725e3_l3.png)
This provides a way to experimentally measure the activation energy of a reaction (the transition state structures are so short-lived that it is often impossible to characterize them experimentally). By measuring k at different temperatures and plotting ln(k) versus 1/T, we can obtain a straight line where slope = –Ea/R.
Activity: Determining Ea
You can estimate the activation energy without constructing an Arrhenius plot if the rate constant was measured at only two temperatures. The slope of an Arrhenius plot is:
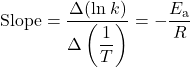
Therefore:
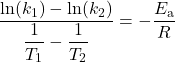
which can be rearranged as:
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

