D12.5 Constitutional Isomers
Compounds with the same molecular formula but different atomic connectivity are called constitutional isomers (or structural isomers). For example, there are two alkanes with the formula C4H10:
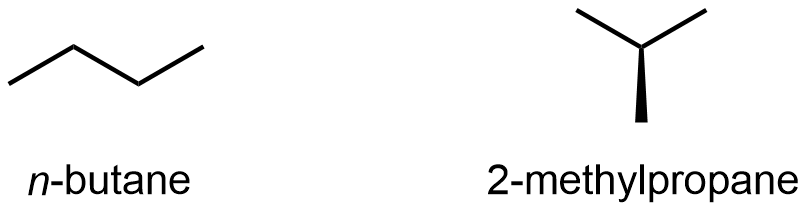
The n– in n-butane stands for normal, meaning an unbranched carbon chain. Typically the “n-” is omitted and the compound is just named “butane”; it is assumed that you know that no prefix refers to the unbranched chain.
The traditional name of 2-methylpropane is isobutane. Often the “2-” is omitted and the compound is just called “methylpropane” because the “2-” position is the only possible location for the methyl group to bond to (if the methyl is bonded to either end, the molecule is n-butane); hence omitting the “2-” is unambiguous here.
Activity: Analyzing Constitutional Isomers
The above activity highlighted that to change from one constitutional isomer to another requires breaking (and re-forming) covalent bonds, which requires significant input of energy. We just saw in the previous section that this high energy demand (~300 kJ/mol) means extremely long timescale for this process to occur at room temperature. In other words, constitutional isomers will not convert from one to another at room temperature. Therefore, they can be synthesized and kept separated: they are different substances.
In addition to examining the connectivity in Lewis structures, another way to tell whether a Lewis structure represents the same molecule as another Lewis structure is to work out the IUPAC names for both structures. IUPAC names are designed so that the same structure always has the same name; if two structures are different, naming them correctly will result in different names.
Exercise: Naming Alkanes
Alkenes and alkynes can also exhibit constitutional isomerism. For example:
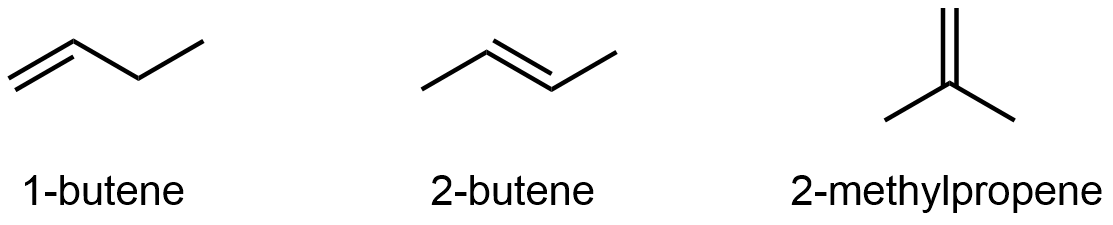
It’s not just the π-bond location that differs; be sure that you can deduce all the covalent bonds that need to be broken and re-formed going from one isomer to another by looking at just the line structures. See the 3D models below to verify.
Exercise: Isomers of Substituted Benzenes
The structure of ortho-xylene is shown here:
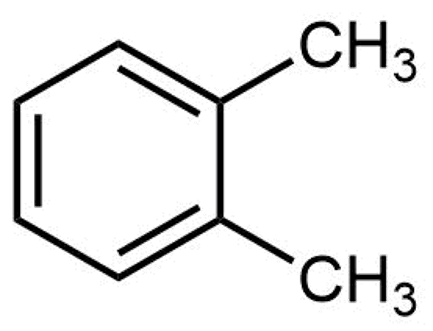
Click on each structure below that is a constitutional isomer of ortho-xylene.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

