D13.1 Molecular Shape Influences London Dispersion Forces
Whether a covalent molecular substance is a solid, liquid, or gas at room temperature (or any other temperature) depends on the strengths of the attractive forces between the molecules that make up the substance. Any attractive forces between molecules are referred to as intermolecular forces (IMFs). Strengths of intermolecular forces vary widely, but IMFs are usually weaker than covalent bonds. One form of IMF, London dispersion forces, has already been discussed in Unit 1, where we used boiling points to indicate the energy needed to overcome intermolecular forces as molecules go from being in close proximity to each other in the liquid phase to being far apart in the gas phase.
The boiling points of linear alkanes (CnH2n+2), with n = 1-20 are shown in the figure below. The boiling points gradually increase with increasing length of the molecule. You should be able to explain this trend in boiling points based on LDFs.
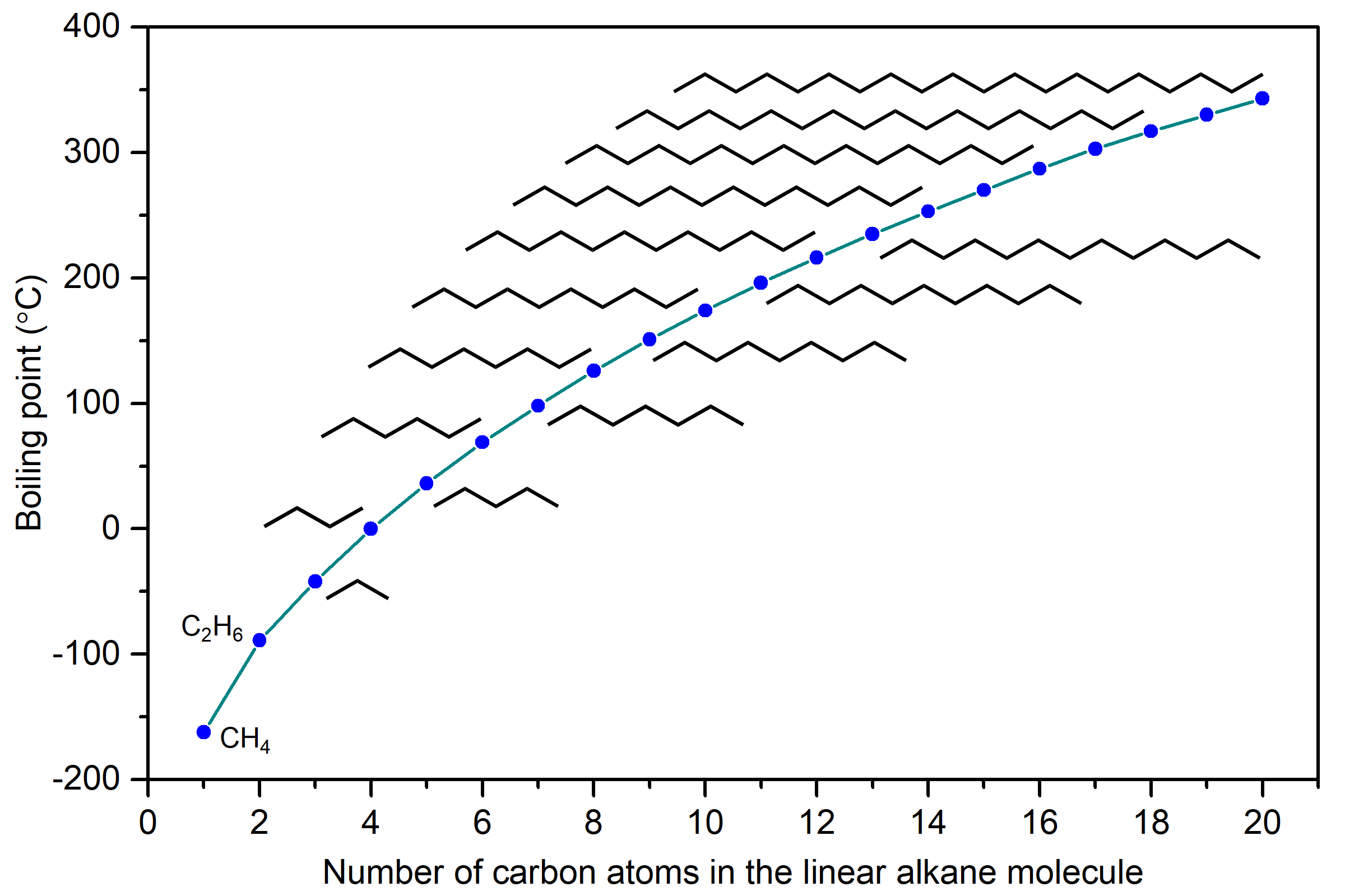
LDFs increase with increasing number of electrons and also with increasing ease of distortion of electron distribution in an atom or molecule. Because longer linear alkanes have more electrons, the LDFs increase and boiling points also increase.
Exercise: Match Boiling Points
Another factor that affects the strength of LDFs is molecular geometry because it influences how effectively electron densities from different molecules can interact. For example, the boiling points for n-pentane, isopentane (2-methylbutane), and neopentane (2,2-dimethylpropane) are 36 °C, 27 °C, and 9.5 °C, respectively, indicating that LDFs are greatest for n-pentane and least for neopentane.
These three molecules are constitutional isomers and therefore have the same number of electrons. In n-pentane, the linear shape provides a greater surface area between molecules when they come in contact, resulting in stronger LDFs between the molecules. Neopentane has the most compact shape of the three—roughly spherical—yielding the smallest surface area for intermolecular contact and, hence, the weakest LDFs.
Exercise: Predicting Boiling Points
Another physical property that is influenced by IMFs is the viscosity of a liquid, which is a measure of the liquid’s resistance to flow. The stronger the IMFs, the more difficult it is for molecules to move past one another, and the greater the viscosity of the liquid. Alkane molecules can get quite large and Figure: Boiling points of linear alkanes shows that the strength of LDFs is quite significant in larger alkanes. (The boiling point of pentadecane C15H32 is 270°C.) Hence, they also have higher viscosity. Some examples of uses for these long-chain alkanes are lubricating oil and paraffin wax. Alkanes with a chain length of 35 or more carbon atoms are found in asphalt (or bitumen), which is a sticky and highly viscous substance.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

