D13.2 Molecular Dipoles; Dipole-Dipole Attractions
Applying Core Ideas: Comparing Propane and Dimethyl Ether
The additional IMF alluded to in the Applying Core Ideas box is called dipole-dipole attraction, which is an attractive electrostatic force between polar molecules. The attractive force arises when the positive end of one molecular dipole interacts with the negative end of another molecular dipole.
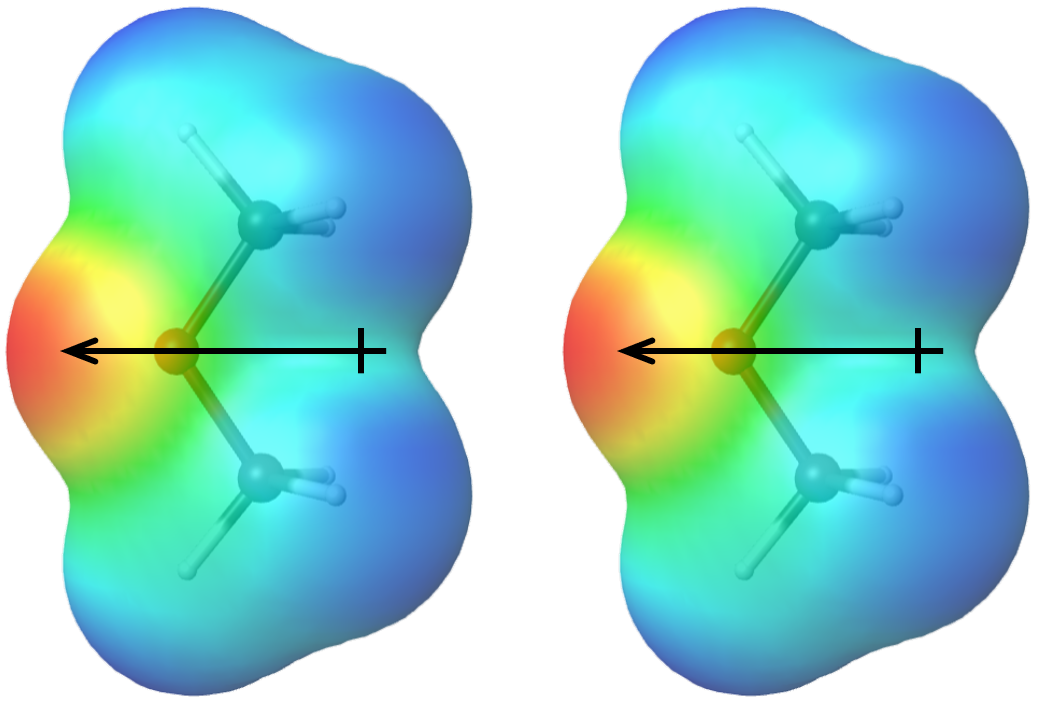
The more polar a molecule is (that is, the larger its molecular dipole moment is), the stronger the dipole-dipole attractions are between molecules of that substance. Molecular polarity depends both on the sizes of the bond dipoles (that is, on electronegativity differences between pairs of bonded atoms) and the shape of the molecule. Physical properties of a substance are influenced by all IMFs between molecules of the substance, so it is important to consider both LDFs and dipole-dipole attractions when predicting properties such as boiling points.
How do we know whether a molecule has a dipole moment? In Section: Bond Polarity we described polar covalent bonds—bonds in which there is an unequal distribution of electron density over two bonded atoms and hence a bond dipole moment. The sum of all bond dipole moments in a molecule gives a molecular dipole moment. Molecules that have a molecular dipole moment are called polar molecules; molecules that have a zero (or near zero) molecular dipole moment are called nonpolar molecules.
To predict whether a molecule is polar, first determine whether there are polar bonds by comparing electronegativities of each pair of bonded atoms. If electronegativity differences are small or zero, there are no polar bonds and the molecule must be nonpolar. If there are polar bonds, the molecule might be polar, but it is also possible that the bond dipoles might cancel.
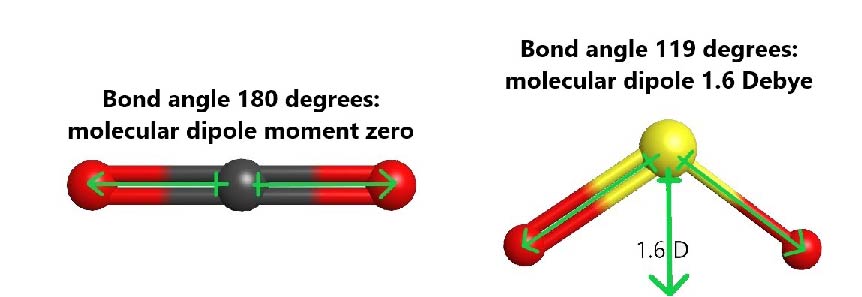
For example, both carbon dioxide (CO2) and sulfur dioxide (SO2) have polar bonds, but only SO2 is polar. In CO2, the central carbon has two σ bonds, it is sp hybridized, and therefore the molecule is linear. The bond dipole of each C=O bond is large (ΔEN = 3.5 − 2.6 = 0.9) and equal in magnitude. However, the two C=O bonds are pointing in exact opposite directions, and result in a molecular dipole moment of zero. In SO2, where the central S atom has two σ bonds and one lone pair, the S atom is sp2 hybridized and the molecule is bent. The sulfur-oxygen bond dipoles are also large (ΔEN = 3.5 − 2.6 = 0.9), but they are situated at an angle and the resultant molecular dipole is not zero.
Bond dipoles behave as vectors, so if you are familiar with vector addition you can predict when bond dipoles cancel and when they do not. Another way to predict is this: molecules with all terminal atoms the same and no lone pairs on the central atom are nonpolar because of cancellation of bond dipoles. (In the case of a molecule with an odd number of electrons, a single electron on the central atom counts as a lone pair.) For multicentered molecules, predicting molecular dipoles is trickier. Generally, if atoms have similar electronegativities, then bond dipoles are weak and the molecular dipole moment is small. For example, because C and H have similar electronegativity, C-H bonds have small bond polarity, and hydrocarbon molecules are nonpolar. The dipole moment of propane, for example, is less than 0.1 D—essentially negligible.
Exercise: Predicting Molecular Polarity
Exercise: Predicting Boiling Points
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

