Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 13 Pre-Class Podia Activity
The boiling point of acetone (MW = 58.08 g/mol) and 2-methylpropane (MW = 58.12 g/mol) are:
| Acetone | 2-methylpropane |
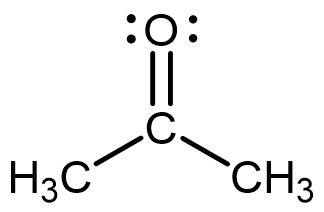 |
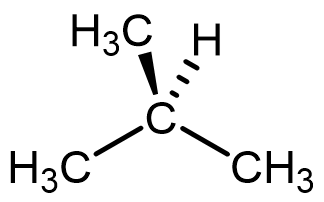 |
| boiling point = 56 °C | boiling point = -12 °C |
Sketch how you imagine three molecules of acetone and three molecules of 2-methylpropane to exist in separate, tiny, closed flasks. Where appropriate, your models should include indications of particle kinetic energy and intermolecular forces between particles. You should use wedge-dash representations to depict matter particles. You can use any notation you wish to depict kinetic energy and intermolecular forces between particles; however, you should clearly indicate the type (or types) of intermolecular forces present in each sample.
Using the models you constructed above, explain the boiling point difference between acetone and 2-methylpropane. Link your models and explanation using reasoning involving Coulomb’s law. What must be true concerning the strength/type(s) of intermolecular forces between molecules of acetone when compared to the strength/type(s) of those between molecules of 2-methylpropane?
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

