Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 14 Pre-Class Podia Activity
Consider two constitutional isomers of C3H9N: trimethylamine and propylamine, bond-line representations of which are provided below.
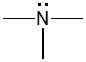 |
|
| trimethylamine | propylamine |
| dipole moment = 0.612 D | dipole moment = 1.19 D |
- In a flask containing one mole of trimethylamine, what intermolecular force(s) would be present?
- In a flask containing one mole of propylamine, what intermolecular force(s) would be present?
- Sketch how you imagine three molecules of trimethylamine to exist in a tiny, closed flask. Illustrate only the strongest intermolecular force for this substance by labeling the location and type of intermolecular force you are drawing.
- Sketch how you imagine three molecules of propylamine to exist in a tiny, closed flask. Show only the strongest intermolecular force for this substance by labeling the location and type of intermolecular force you are drawing.
- Predict which compound would have the higher boiling point. Discuss the features of your trimethylamine/propylamine models that were crucial for making this prediction. Provide reasoning that links these features to the concept of energy using Coulomb’s law.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

