D34.4 pH and pOH
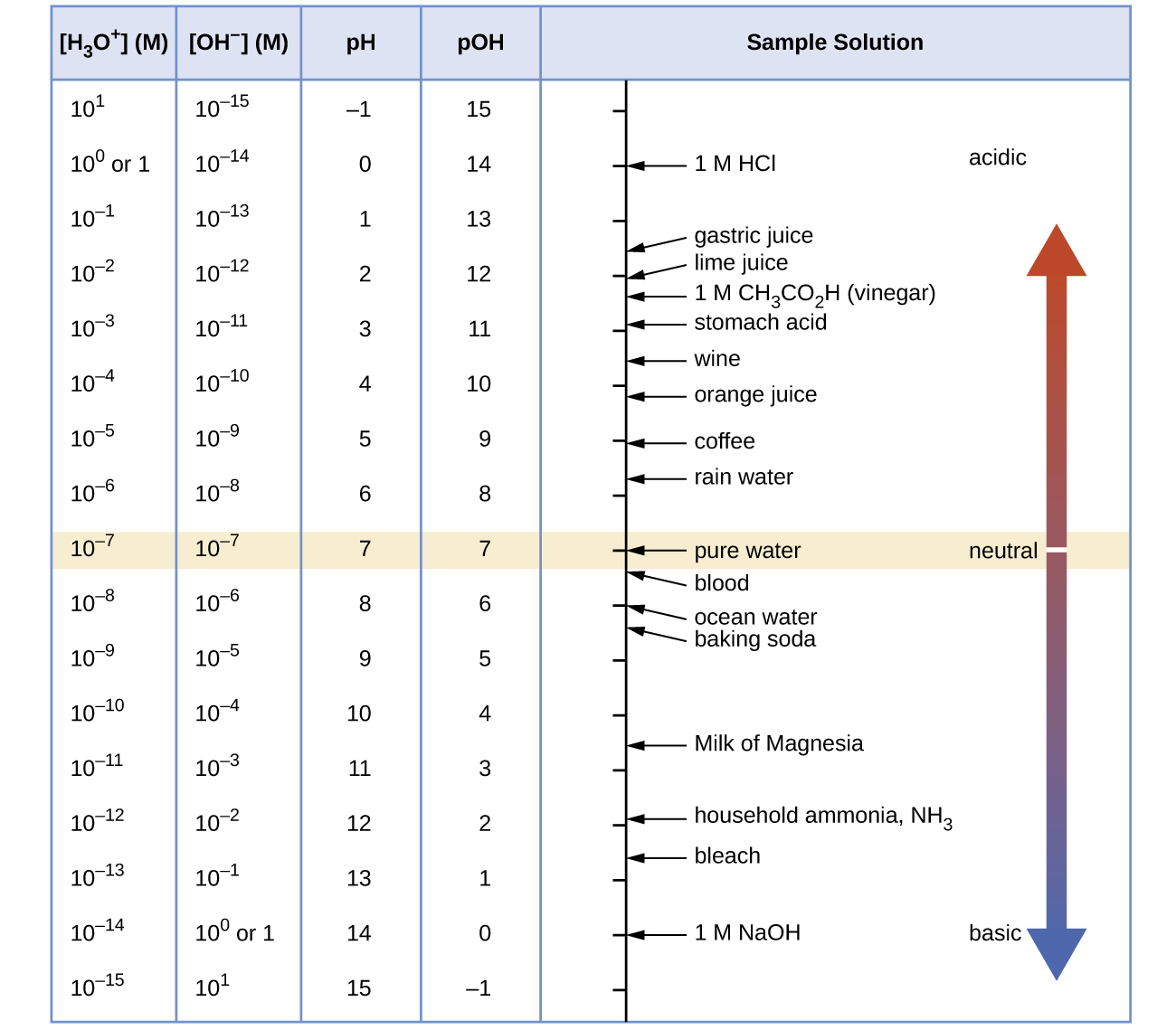
The concentrations of H3O+ and OH– in a solution are important for the solution’s acid-base properties and often affect the chemical behaviors of other solutes. A solution is neutral if its [H3O+] = [OH–]; acidic if its [H3O+] > [OH–]; and basic if its [H3O+] < [OH–].
A common means of expressing values that span many orders of magnitude is to use a logarithmic scale. One such scale is based on the p-function:
where “X” is the quantity of interest and “log” is the base-10 logarithm. The pH of a solution is therefore defined as:
The reason for dividing by the units “mol/L” (M) is that [H3O+] has units of mol/L and taking the logarithm of a unit makes no sense. From here on we will assume that you are aware that only the numeric value of a concentration (or other quantity) needs to be used as the argument of a logarithm and we will not explicitly divide by the units.
If a pH value is known, the concentration of hydronium ions can be calculated:
Here we assume that you know that units are required for the concentration obtained from this equation and the units are mol/L.
The hydroxide ion concentration may be similarly expressed as pOH:
Finally, the relation between pH and pOH can be derived from the Kw expression:
| Kw | = | [H3O+][OH–] |
| -log(Kw) | = | -log([H3O+][OH–]) |
| pKw | = | -log([H3O+]) + (-log([OH–])) |
| pKw | = | pH + pOH |
At 25 °C:
Therefore, at this temperature:
| Classification | Relative Ion Concentrations | pH at 25 °C |
|---|---|---|
| acidic | [H3O+] > [OH−] | < 7 |
| neutral | [H3O+] = [OH−] | 7 |
| basic | [H3O+] < [OH−] | > 7 |
Because Kw is temperature dependent, the correlations between pH values and the acidic/neutral/basic adjectives varies at different temperatures. For example, [H3O+] in pure water at 80 °C is 4.9 × 10−7 M, which corresponds to pH and pOH values of:
pOH = -log[OH–] = -log(4.9 × 10−7) = 6.31
At this temperature, neutral solutions have pH = pOH = 6.31, acidic solutions have pH < 6.31 and pOH > 6.31, and basic solutions have pH > 6.31 and pOH < 6.31. This distinction can be important when studying certain processes that occur at temperatures other than 25 °C, such as acid-base reactions in the human body where temperatures are typically 37 °C.
Unless otherwise noted, references to pH values are presumed to be those at 25 °C. The table below shows the relationships among [H3O+], [OH−], pH, and pOH, and gives these values for some common substances.
Activity: pH and Relative Strengths of Acids
Exercise: pH of Aqueous Solutions
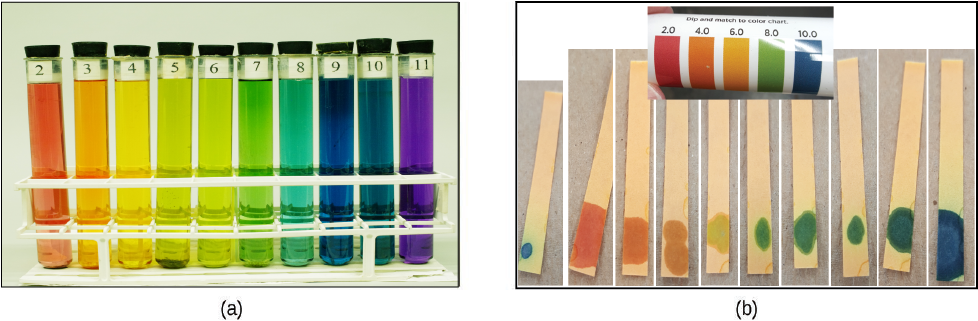
The acidity of a solution is typically determined by measuring its pH. The pOH of a solution is not usually measured, but it is easily calculated from an experimentally determined pH value. The pH of a solution can be directly measured using a pH meter or visually estimated using colored indicators.

Activity: Determining Ka
Activity: Using Ka to Calculate Concentrations
The percent ionization of a weak acid is another measure of the strength of an acid, HA:
A stronger acid, with a larger Ka, has higher percent ionization than a weaker acid (for the same concentration). The percent ionization for a solution of a weak acid is concentration dependent: it increases with decreasing acid concentration.
Exercise 4: Percent Ionization
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

