Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your answer.
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time.
Day 26 Pre-Class Podia Activity
In last week’s lab, you synthesized acetaminophen and in a previous Podia activity, you thought about (from an electrostatics standpoint) how acetic acid and 4-aminophenol must collide to form acetaminophen.
Notice, however, that there are two centers of negative charge on 4-aminophenol: on the N atom and on the O atom. So, these molecules can collide in two ways to produce two different products, an amide (acetaminophen) and an ester:
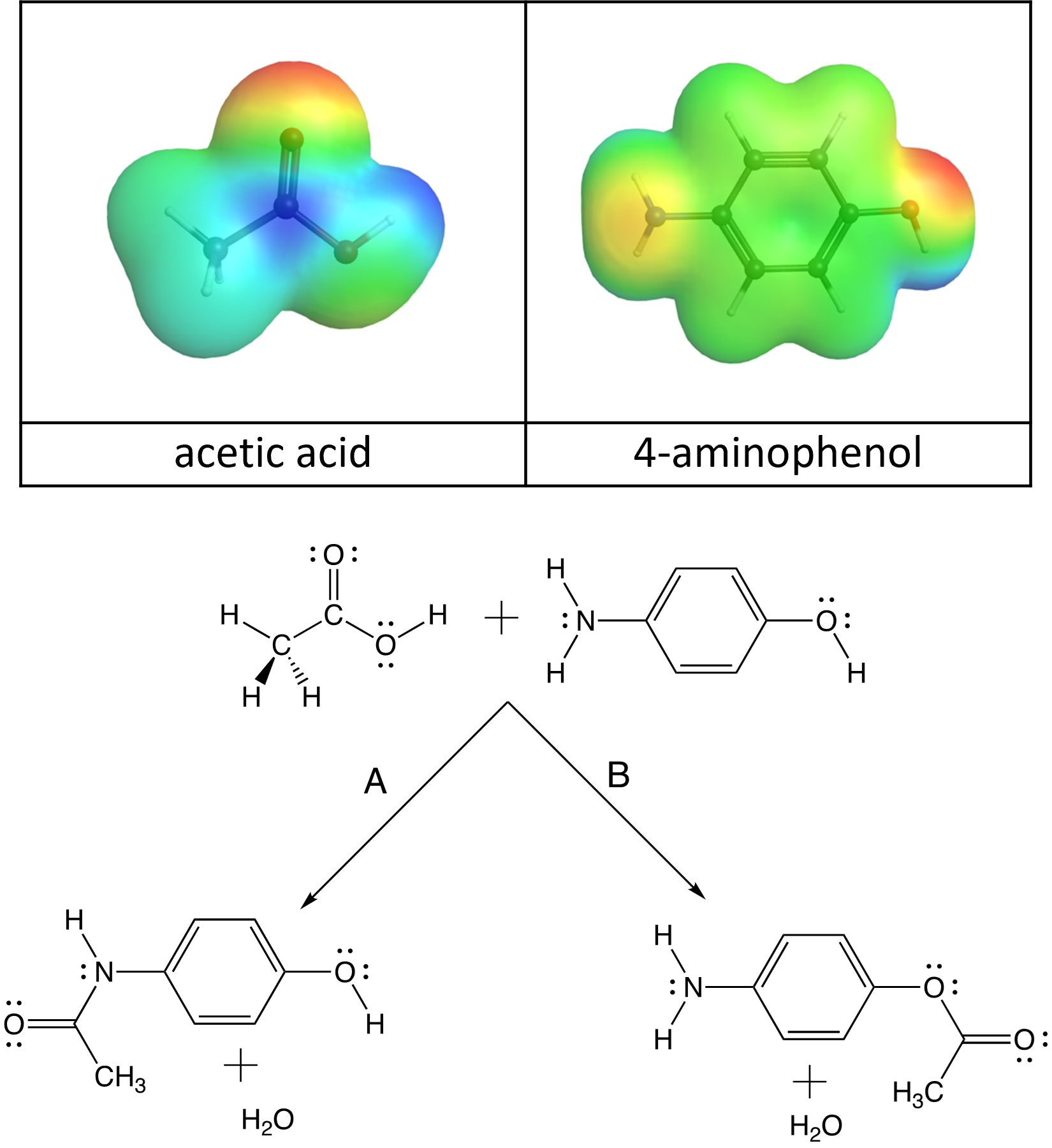
- It’s a bit hard to tell from these electrostatic potential diagrams, but the partial charge on the O atom is more negative than the N atom. Based on this, which reaction path (A or B) would you expect to have the lower activation energy and occur more quickly?
- Even though electron delocalization occurs in both functional groups, this stabilizing phenomenon occurs to a greater extent in amides, when other structural factors are equal. Based on this which reaction path (A or B) would you expect to have a more negative Gibbs free energy change?
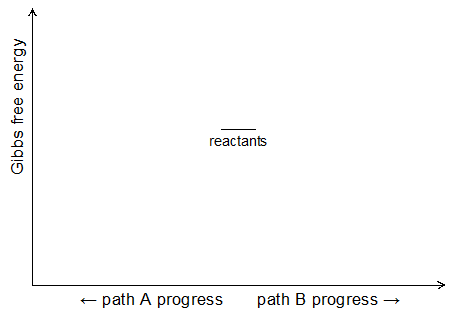
- Using the diagram below, build reaction progress diagrams that represent the Gibbs energy changes as acetic acid and 4-aminophenol transform into two different sets products. (Plot the products energy of path A to the left and the products energy of path B to the right. Use your diagram to justify why you synthesized acetaminophen at elevated temperature.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

