D39.3 Acid-Base Indicators
Acid-base indicators are substances with intense colors that change color when [H3O+] reaches a particular value. Acid-base indicators are either weak organic acids or weak organic bases and can be used to determine the pH of a solution.
For example, the equilibrium in a solution of the acid-base indicator methyl orange, a weak acid, can be represented by an equation in which we use “HIn” as a simple representation for the complex methyl orange molecule:
![Rendered by QuickLaTeX.com \begin{array}{ccccccc} \text{HIn}(aq) & + & \text{H}_2\text{O}(l) & {\rightleftharpoons} & \text{H}_3\text{O}^{+}(aq) & + & \text{In}^{-}(aq) \\[0.5em] \text{red} & & & & & & \text{yellow} \end{array}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-d030bb716692898699e85a7782ff5045_l3.png)
If we add an acid to a solution containing methyl orange, the increased [H3O+] will result in more HIn (the nonionized red form) at equilibrium. If we add a base, more In– (the anionic yellow form) will be present. The overall color of the solution is the visible result of the ratio of the concentrations of the two species: if most of the indicator is present as In−, then we see the color yellow; if most is present as HIn, then we see the color red.
To consider this more quantitatively, we can rearrange the equation for Ka and write:
When [H3O+] = Ka,HIn, 50% of the indicator is present in the red form (HIn) and 50% is in the yellow ionic form (In–), and the solution overall appears orange in color. When [H3O+] increases to 8 × 10−4 M (pH = 3.1), the solution turns red. No significant change in color is visible for further increase in [H3O+]. When [H3O+] decreases to 4 × 10−5 M (pH = 4.4), most of the indicator is in the yellow ionic form, and further decrease in [H3O+] does not produce a visible color change. The pH range of 3.1 – 4.4 is the color-change interval of methyl orange, the range of pH values over which the color change takes place.
There are many different acid-base indicators. Their color-change intervals have a wide range of pH values (see figure below). Universal indicators and pH paper contain a mixture of indicators and exhibit different colors at different pH values.
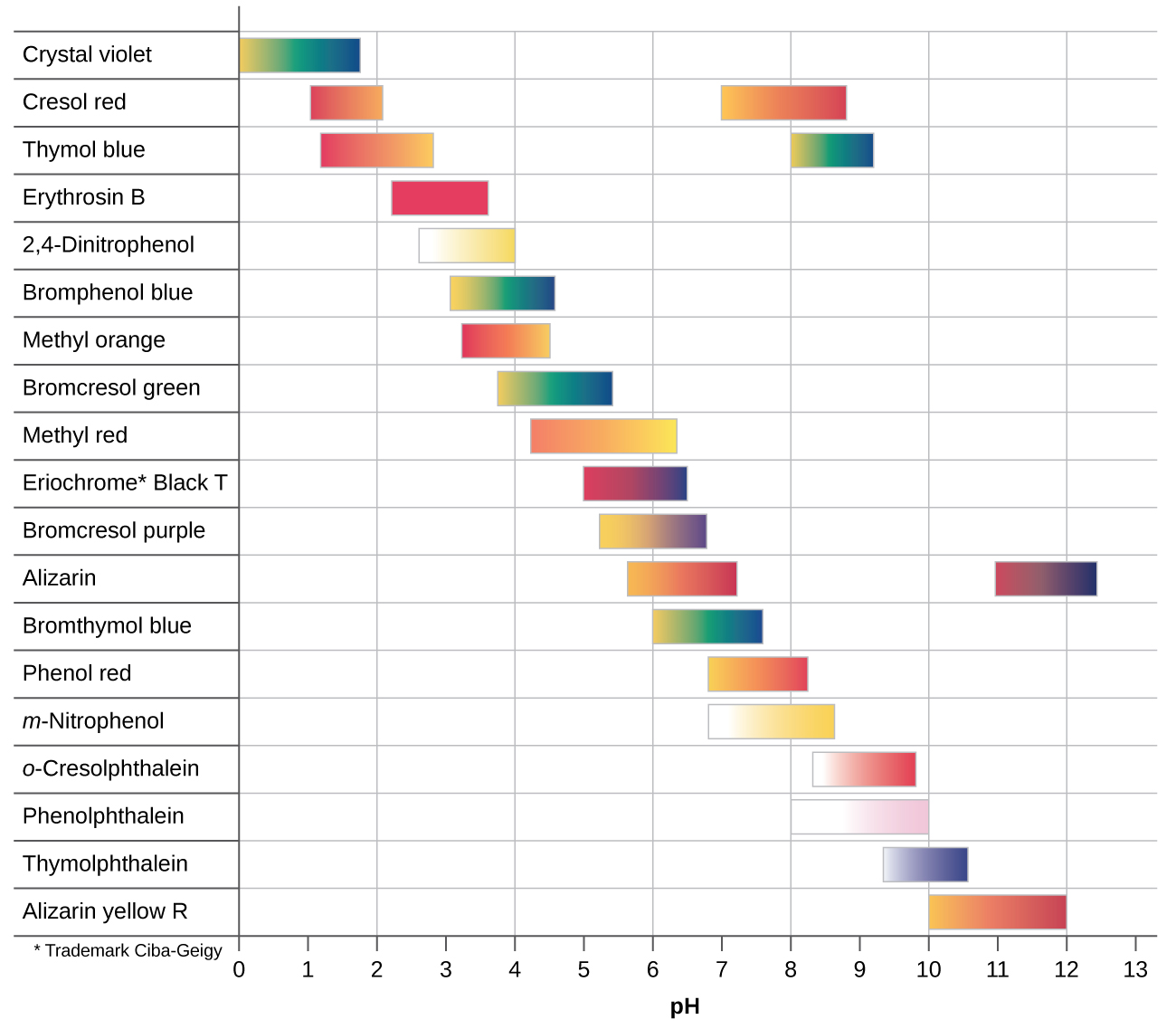
Titration curves can help in selecting an indicator that will provide a sharp color change at the equivalence point. The best selection is an indicator with a color-change interval that brackets the pH at the equivalence point of the titration. (We can also base our choice of indicator on the calculated pH at the equivalence point.)
The color-change intervals of three indicators are shown in Figure: Indicator Choices. The steep section of the titration curves of HCl and of CH3COOH spans the color-change interval of phenolphthalein. We can use phenolphthalein for titrations of either acid.
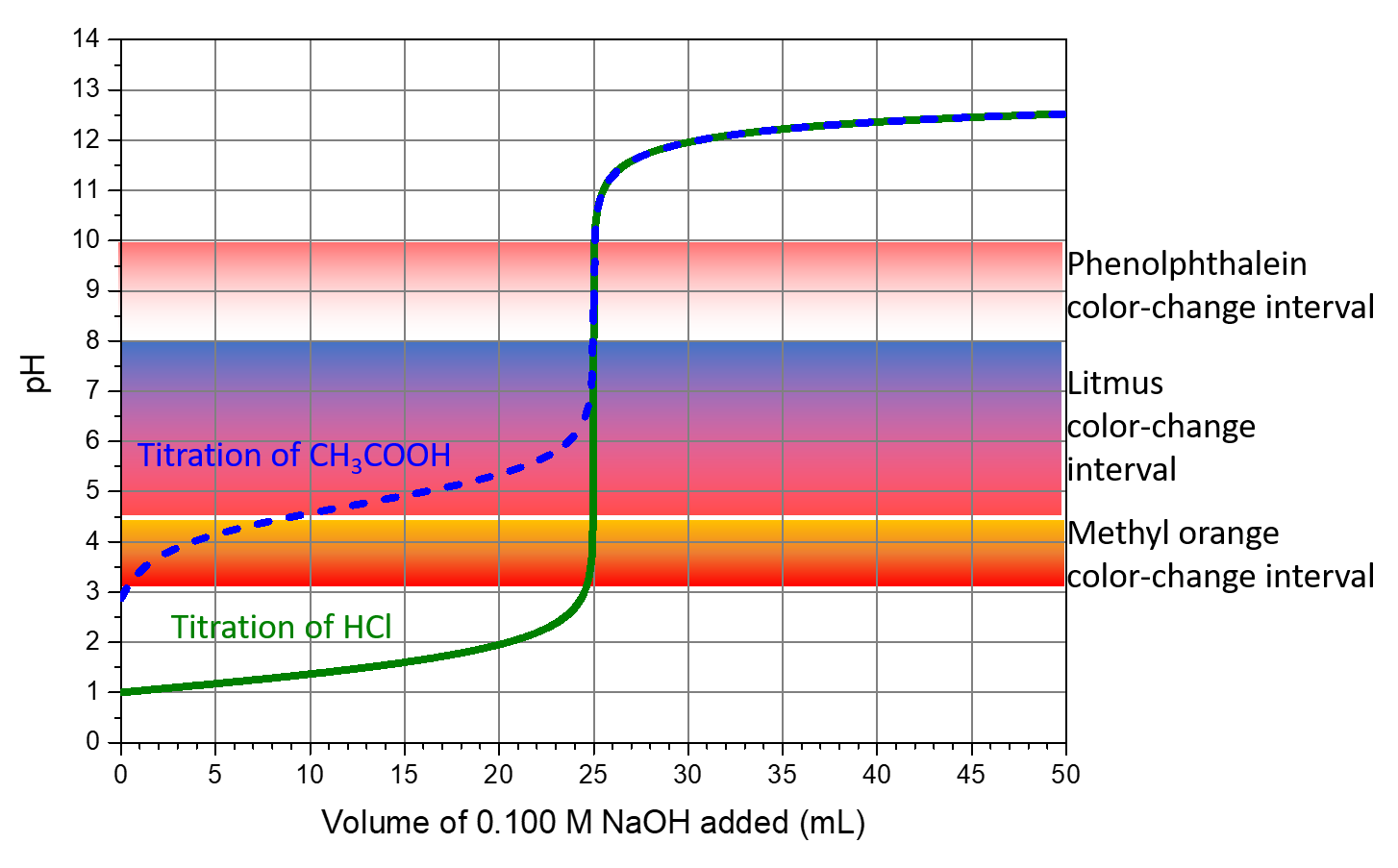
Litmus is a suitable indicator for the HCl titration. However, we should not use it for the CH3COOH titration because the solution pH is within the color-change interval when only ~8 mL of NaOH has been added, and it does not leave the range until 25 mL has been added. The color change would be very gradual, taking place during the addition of ~17 mL of NaOH, making litmus useless as an indicator of the equivalence point.
We can use methyl orange for the HCl titration, but it would not give very accurate results: (1) It completes its color change slightly before the equivalence point is reached (but very close to it, so this is not too serious); (2) it changes color during the addition of 0.5 mL of NaOH, which is not so sharp a color change as that of litmus or phenolphthalein. Methyl orange would be completely useless as an indicator for the CH3COOH titration. Its color change is completed long before the equivalence point is reached and hence provides no indication of the equivalence point.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

