D28.3 Bimolecular Elementary Reactions
The collision and reaction of two molecules or atoms in an elementary reaction is a bimolecular elementary reaction. There are two general types of bimolecular elementary reactions. In one type, the two reactants are different:
The rate for such a reaction is directly proportional to concentration of A as well as directly proportional to concentration of B:
In the other type of bimolecular elementary reaction, the two reactants are the same:
The rate for such a reaction is proportional to the square of concentration of A:
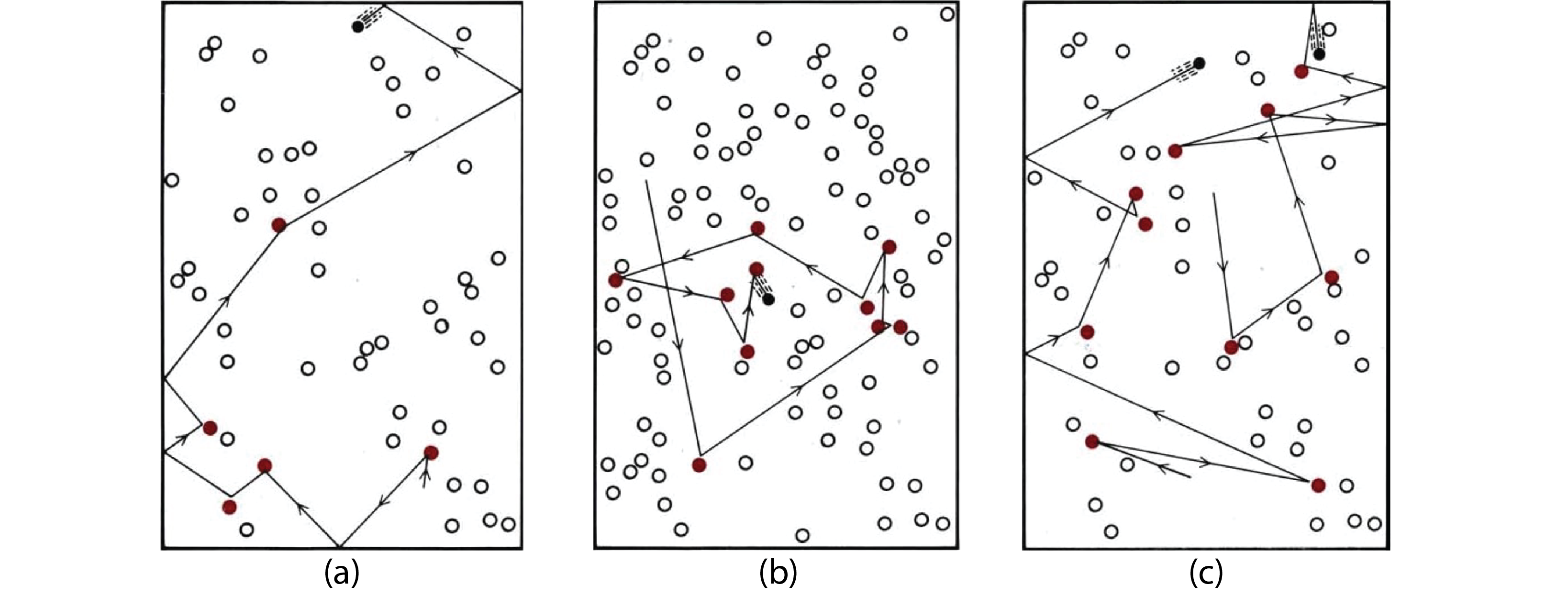
Consider the first case above, A + B ⟶ products. How does the concentration of each reactant affect the number of collisions, and hence the rate of the reaction? In the figure below, reactant A is shown in black and reactant B in white. We see that the number of collisions doubles when the number (and concentration) of either reactant doubles. Assuming that the fraction of collisions that results in reaction is the same in all three scenarios, this means that the rate of reaction doubles when the concentration of either reactant doubles.
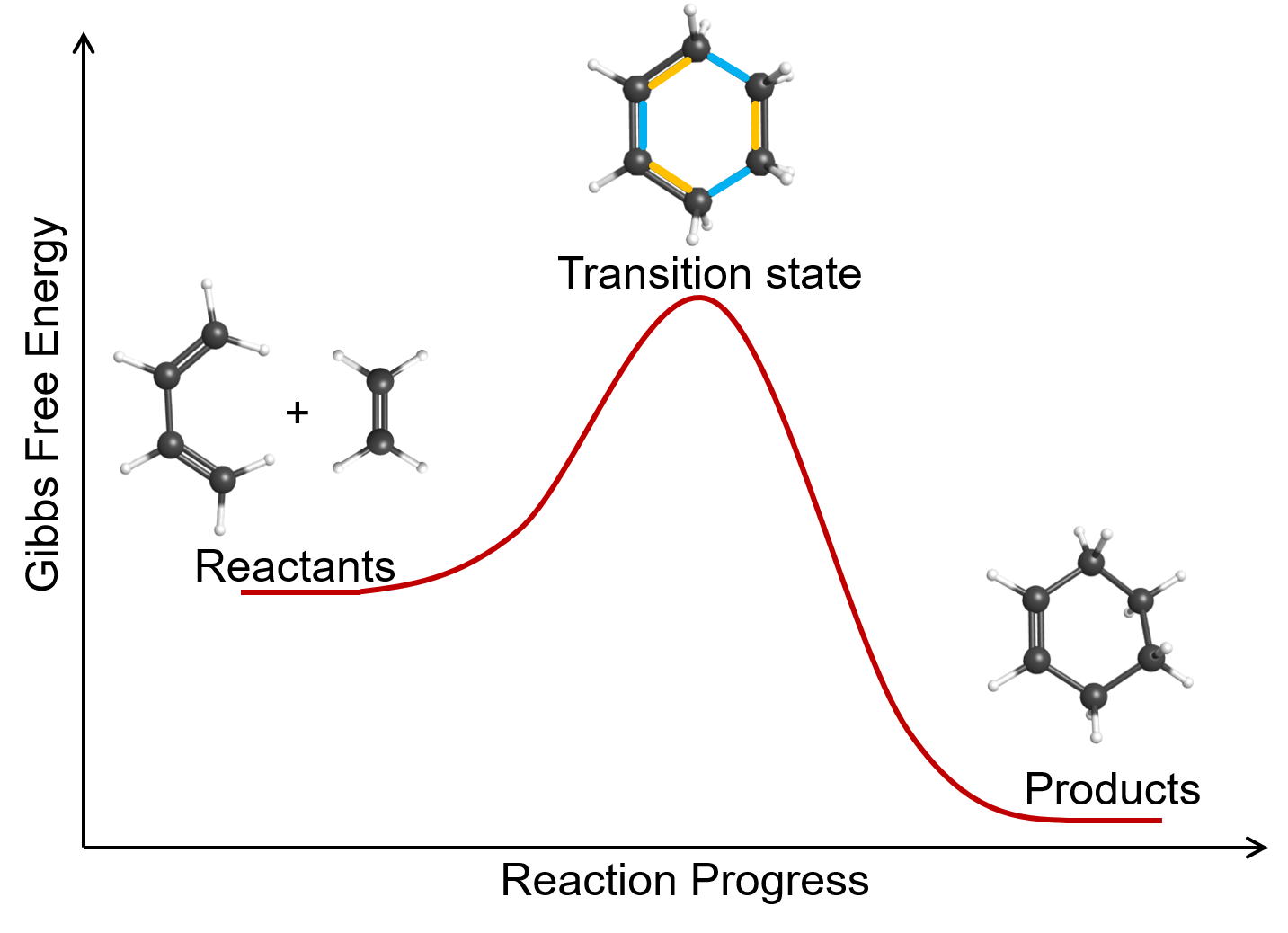
An example of a bimolecular elementary reaction is the gas-phase reaction of 1,3-butadiene with ethene to form cyclohexene:
Exercise: Bimolecular Reactions
Exercise: Steric Factor
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

