D30.5 Homogeneous Catalysis
Reactions that are facilitated by catalysts can be divided into two classes: homogeneous catalysis and heterogeneous catalysis. A homogeneous catalyst is present in the same phase as the reactants. We have already discussed several examples where catalyst and reactants are all in the same phase.
Another example is acid-catalyzed decomposition of methyl acetate to form acetic acid and methanol, a type of ester hydrolysis. (Hydrolysis is the reverse reaction of a condensation reaction that produces water as the byproduct.) This is a homogeneous catalytic reaction because reactants, products, and catalyst are all in aqueous solution.
The reaction mechanism is shown below. Each step in the mechanism is an acid-base reaction, a type of reaction that we will learn about in more detail in the next unit.
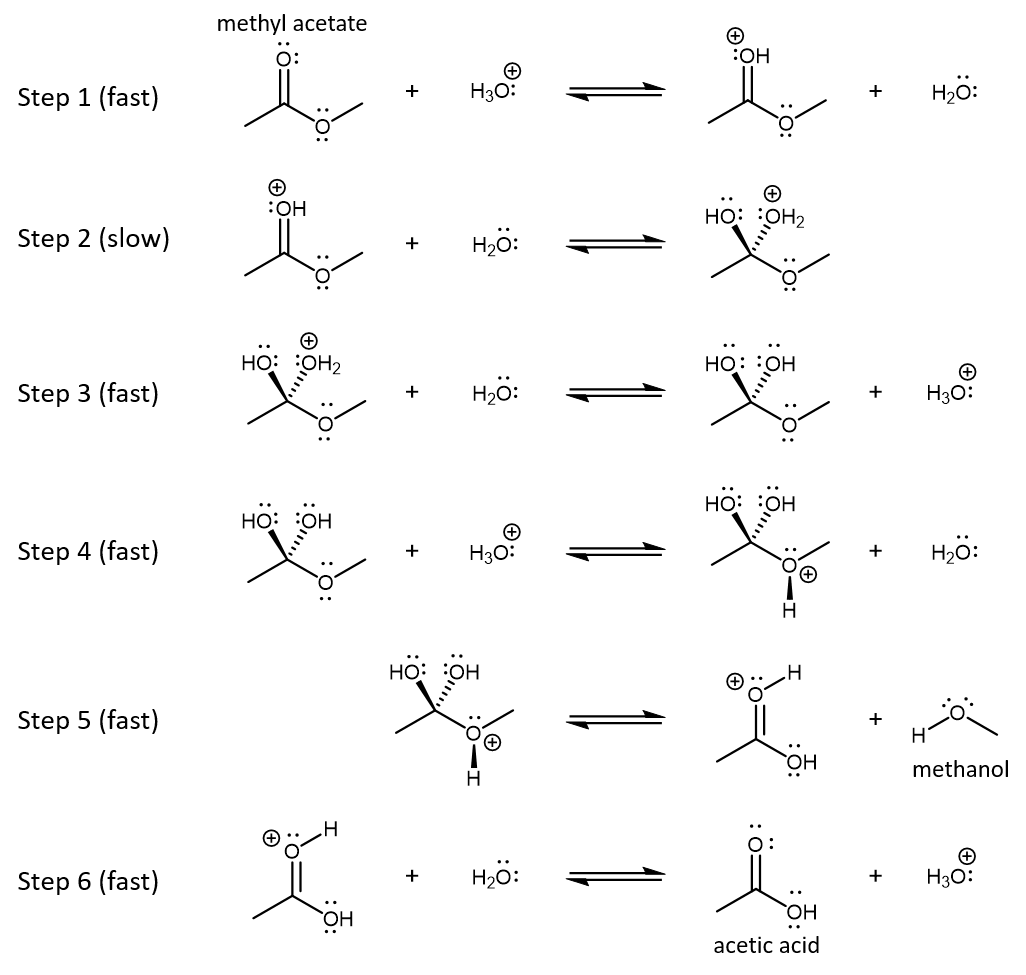
Activity: Analyzing a Reaction Mechanism
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

