D31.1 Heterogeneous Catalysts
A heterogeneous catalyst is present in a different phase from the reactants. Such catalysts are usually solids, and often function by furnishing an active surface upon which one or more steps in the reaction can occur.
A heterogeneous catalytic reaction has at least four steps in its reaction mechanism:
- Adsorption of the reactant(s) onto the surface of the catalyst
- Activation of the adsorbed reactant(s)
- Reaction of the adsorbed reactant(s)
- Diffusion of the product(s) from the surface into the gas or liquid phase (desorption)
Any one of these steps may be slow and thus may serve as the rate determining step. But the overall rate of the reaction is still faster than it would be without the catalyst. The figure below illustrates catalysis the reaction of an alkene with hydrogen on the surface of a nickel catalyst. The overall reaction is
![]()
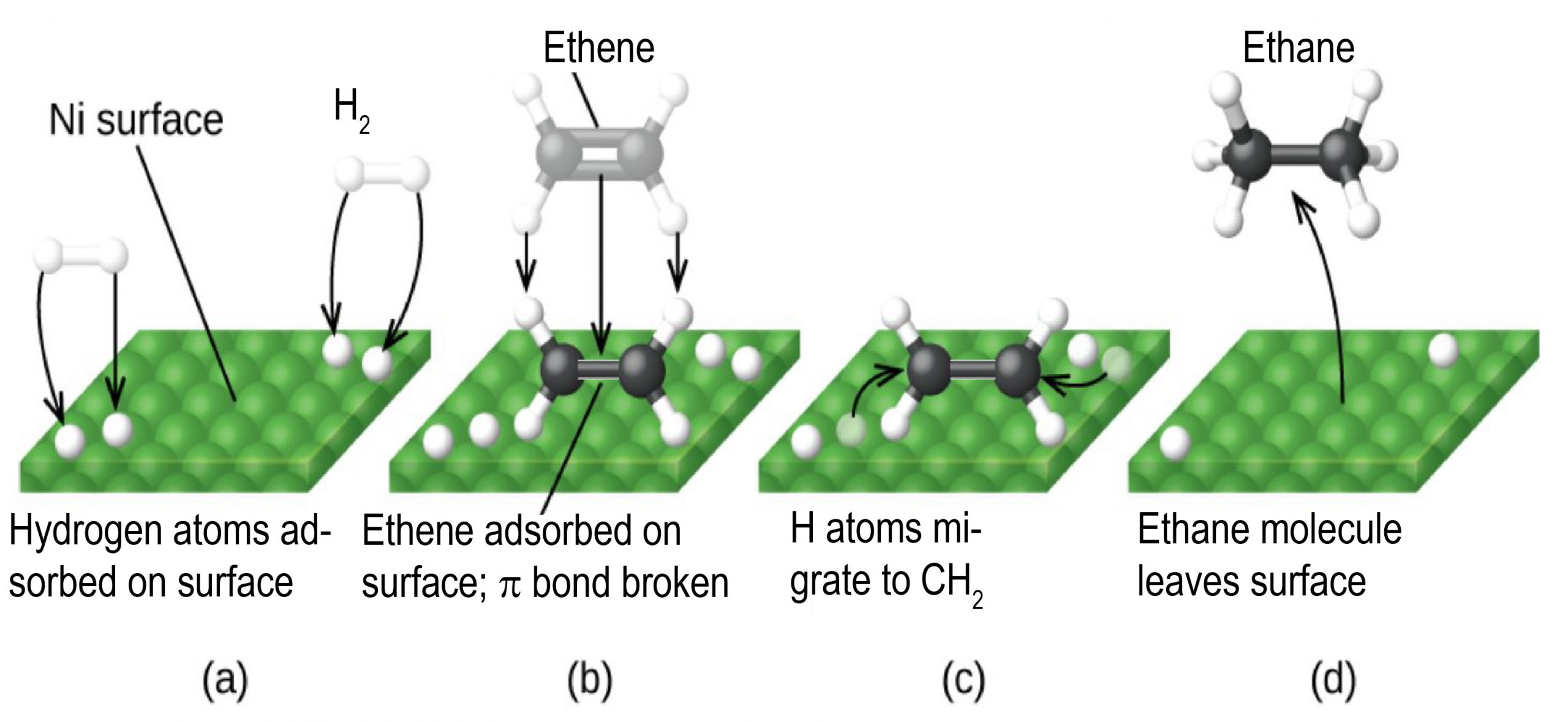
The uncatalyzed C2H4(g) + H2(g) ⟶ C2H6(g) reaction would necessitate a transition state where the C=C π bond and the H-H σ bond are breaking while the two C-H σ bonds form. Such a transition state is so high in energy that without a catalyst, H2 is unreactive towards alkenes under most conditions.
Nickel is a catalyst often used in the hydrogenation of polyunsaturated fats and oils to produce saturated fats and oils. Other significant industrial processes that involve the use of heterogeneous catalysts include the preparation of sulfuric acid, the synthesis of ammonia from nitrogen and hydrogen, the oxidation of ammonia to nitric acid, and the synthesis of methanol. Heterogeneous catalysts are also used in the catalytic converters found on most gasoline-powered automobiles.
Exercise: Catalysis and Reaction Energy Diagram
Exercise: Catalytic Mechanisms
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

