D36.2 Amino Acids
Structure
Amino acids are amphiprotic because each amino acid molecule contains a carboxylic acid group that can donate a proton and an amine group that can accept a proton. Carboxylic acids are moderately acidic, many with Ka of ~10-5. Organic amines are somewhat basic, many with Kb of ~10-4. This combination creates an interesting situation: there are two conjugate acid-conjugate base pairs, so there are four possible structures. These are carboxylic acid protonated and amine group protonated, carboxylic acid deprotonated and amine group protonated, carboxylic acid protonated and amine group deprotonated, and carboxylic acid deprotonated and amine group deprotonated. Which of these situations is most important depends on pH and on the sizes of Ka or Kb for each group. The figure below shows the values for a typical amino acid.

The carboxylic acid group, with Ka = 10-5, is a stronger acid than the protonated amine group, with Ka = Kw/Kb(amine) = 10-14/10-4 = 10-10. The amine group (Kb = 10-4) is a stronger base than the carboxylate anion (Kb = 10-9). You can use the methods developed in the preceding section to calculate whether each group is protonated or deprotonated at a given pH.
Exercise: Amino Acid Protonation in Neutral Solution
Based on your calculations, at the pH of a typical living organism, an amino acid exists as a zwitterion (German for “double ion”), a species with no overall electrical charge but with separate parts that are positively and negatively charged.
The formation of a zwitterion is analogous to the acid-base reaction between methylamine (Kb = 4.4 × 10-4) and acetic acid (Ka = 1.8 × 10-5):
where the equilibrium favors products because:
Increasing the pH of an amino acid solution by adding hydroxide ions can remove the hydrogen ion from the -NH3+ group:
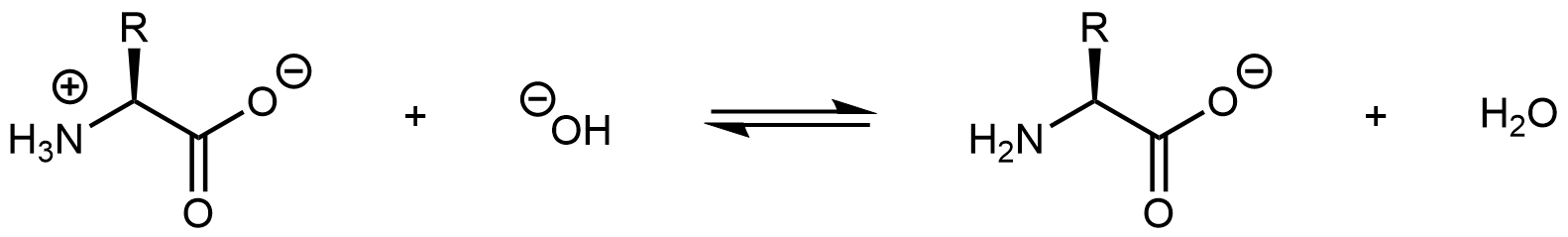
The product molecule is no longer a zwitterion. Instead, it is an anion with an overall charge of -1.
Similarly, decreasing the pH by adding strong acid to an amino acid solution protonates the -COO– part of the zwitterion:
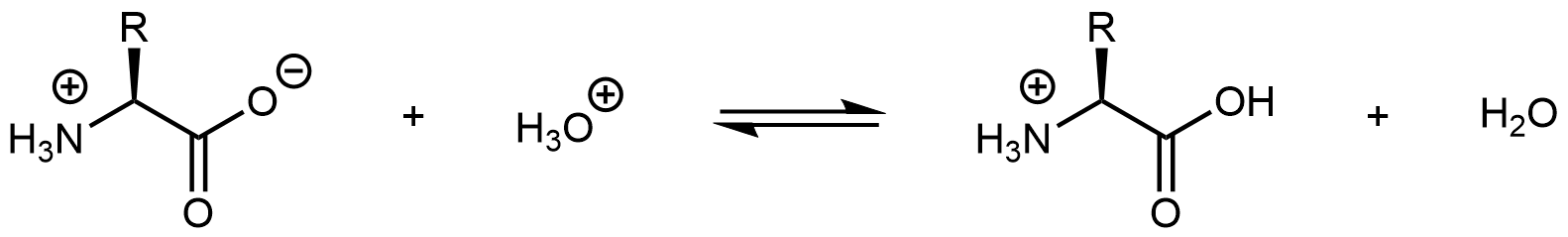
Again, the product molecule is not a zwitterion, but a cation with an overall charge of +1.
Side Chain
The backbone amine and carboxylic acid groups undergo condensation reactions to form amide linkages when amino acids polymerize to form proteins. Therefore, in a strand of protein, the side chain “R” group of each amino acid branches off the protein backbone and can influence protein 3D structure. Knowing the nature of these side chains is important for understanding protein structure.
We have previously grouped the twenty amino acids found in human proteins into two groups—nonpolar/hydrophobic and polar/hydrophilic—depending on the nature of their side chain. Now, we can further divide the latter group into three groups: polar but neutral, basic, and acidic.
Polar side chain
The six amino acids shown in below have side chains that are polar, but neither acidic nor basic.
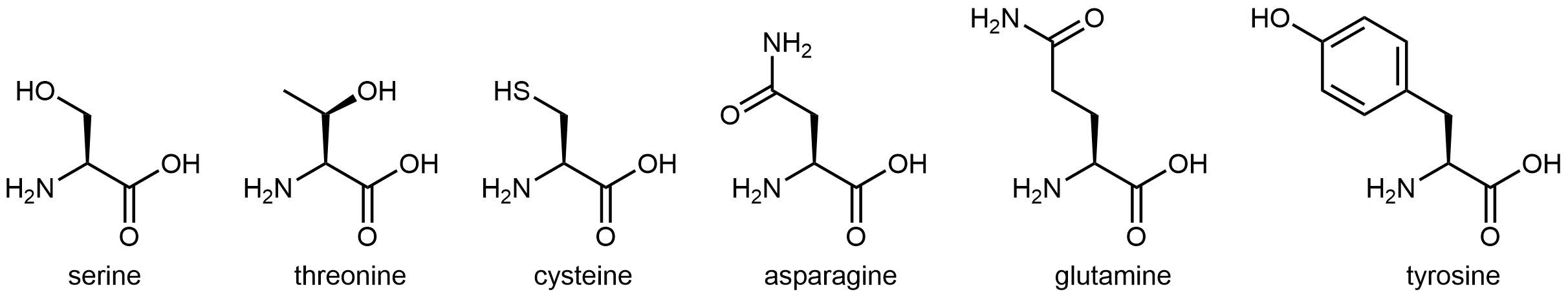
All of these amino acids have a side chain that contains a functional group with significant dipole moment. Five of them can also form strong hydrogen-bonding interactions with water molecules (containing either an alcohol group or an amide group). These characteristics make these side chains hydrophilic, but neither the alcohol group nor the amide group are particularly acidic or basic.
Cysteine has a thiol (-SH) functional group. While it does not form strong hydrogen bonds with water molecules, it does impart a dipole moment to this relatively small side chain, making this side chain overall polar. The thiol functional group is more acidic than the alcohol functional group, but its Ka is still fairly low, ~10-10.
Basic side chain
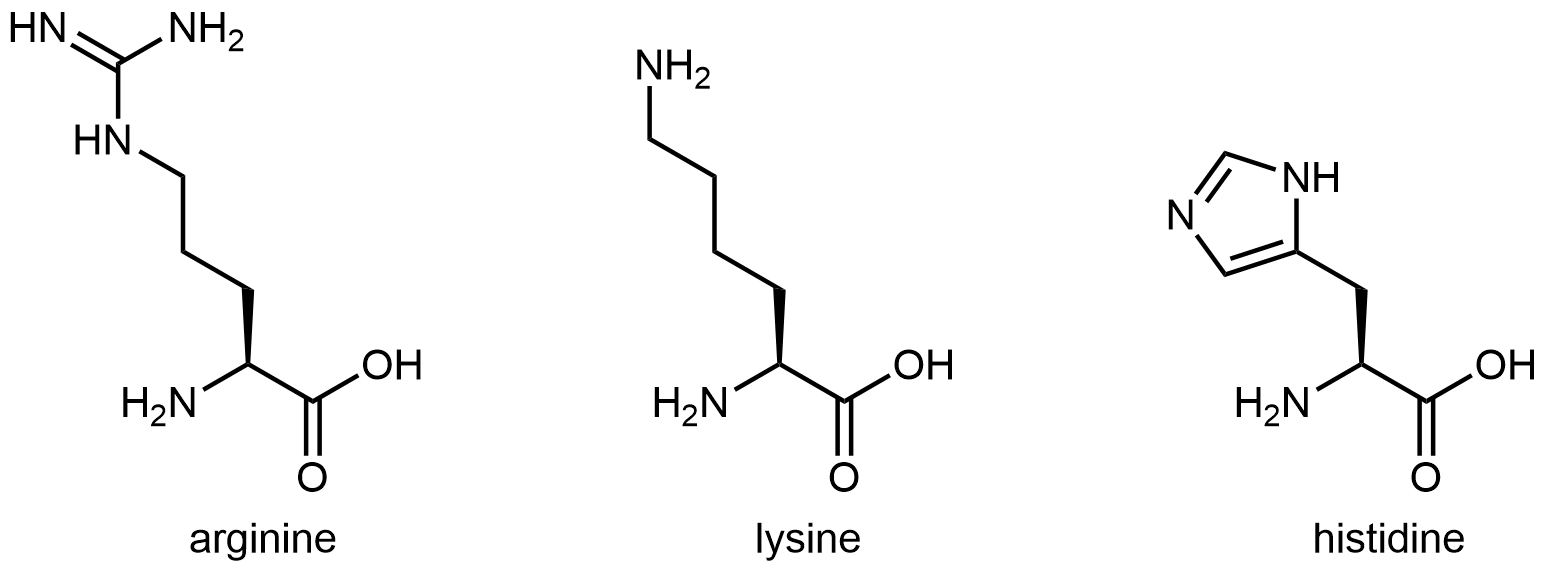
The three amino acids below have side chains containing functional groups that are basic:
Activity: What Part of the Histidine Side Chain Is Most Basic?
The amine functional group at the end of the side chain in lysine imparts basic characteristics to that side chain. The functional group in arginine’s side chain is more complex, but similar to the analysis in the activity above for histidine, you can determine which nitrogen is more important in making the side chain basic by drawing and analyzing a set of resonance structures.
Acidic side chain
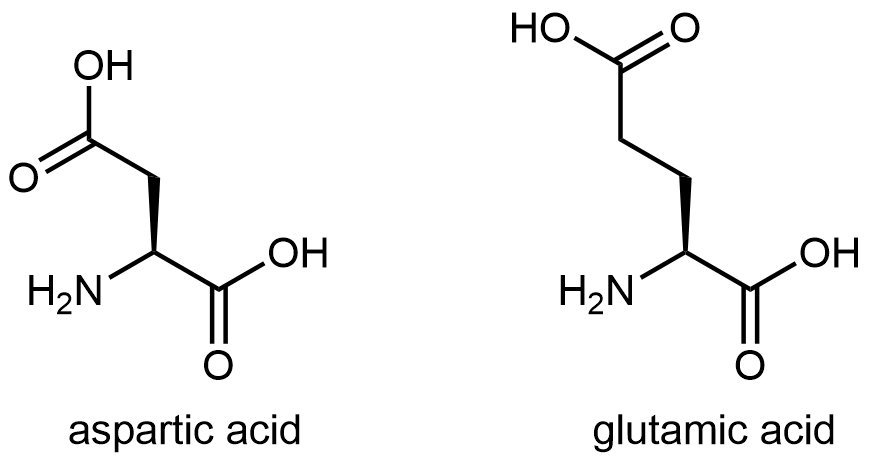
The figure below shows two amino acids that have an acidic side chain containing the carboxylic acid functional group:
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

