D34.2 Autoionization of Water
H2O is the base of its conjugate acid H3O+, and H2O is also the acid of its conjugate base OH–. (However, H3O+ is not the conjugate acid of OH–; these two species are not a conjugate acid-base pair because their structures do not differ by a single H+.)
Hence, H2O can react as an acid or a base depending on the other species involved in the reaction. In pure water, H2O acts as both acid and base—a very small fraction of water molecules donate protons to other water molecules:
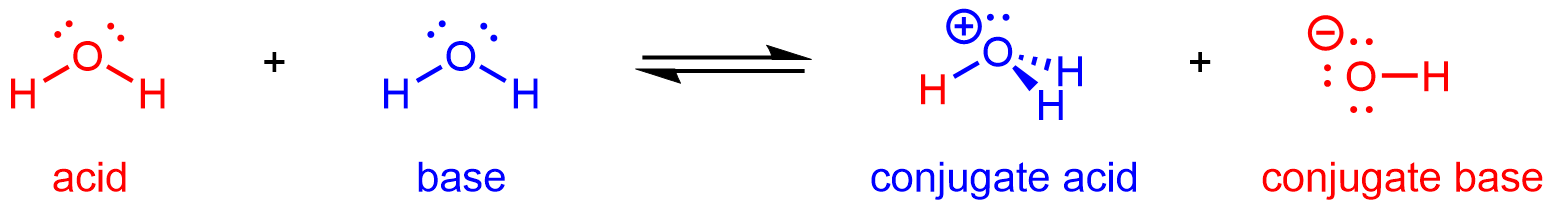
This type of reaction, in which a substance ionizes when one molecule of the substance reacts with another molecule of the same substance, is referred to as autoionization.
Pure water undergoes autoionization to a very slight extent at 25 °C. The equilibrium concentrations of H3O+ and OH– give an autoionization constant for water, Kw = 1.0 × 10−14 at 25 °C.
Because it is the mathematical product of concentrations of two ions, it is also called the ion-product constant for water.
Activity: Autoionization of Water
Exercise: Autoionization of Water
Water is an example of an amphiprotic chemical species, a molecule that can either gain a proton or lose a proton in a Brønsted-Lowry acid-base reaction. Amphiprotic species are also amphoteric, a more general term for a species that may act either as an acid or a base by any definition (not just the Brønsted-Lowry definition). For example, the bicarbonate ion is also amphoteric:
HCO3–(aq) + H2O(ℓ) ⇌ H2CO3(aq) + OH–(aq)
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

